About Livermorium
Livermorium (formerly known as Ununhexium, symbol: Uuh) is a synthetic superheavy element first produced in 2000 by the Joint Institute for Nuclear Research (JINR) in Dubna, Russia. A team from Lawrence Livermore National Laboratory also worked on the element, and though they gave credit to the JINR group for the discovery, the IUPAC rejected the name moscovium for the element in favor of recognizing the Lawrence Livermore laboratory with the element's name. To date, very few atoms of livermorium have been produced, precluding both in-depth study of the element's properties and well as the development of practical applications for it. As the heaviest member of group 16 in the periodic table, livermorium would likely exhibit similar chemical properties to other members of this group, showing the most similarity to its nearest neighbor, polonium.
Livermorium Properties
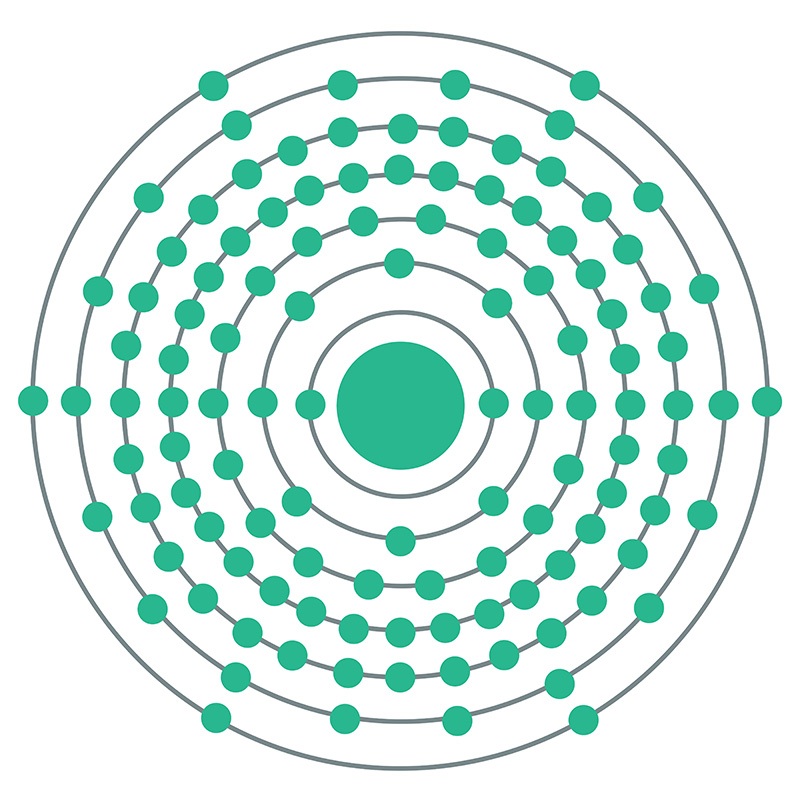
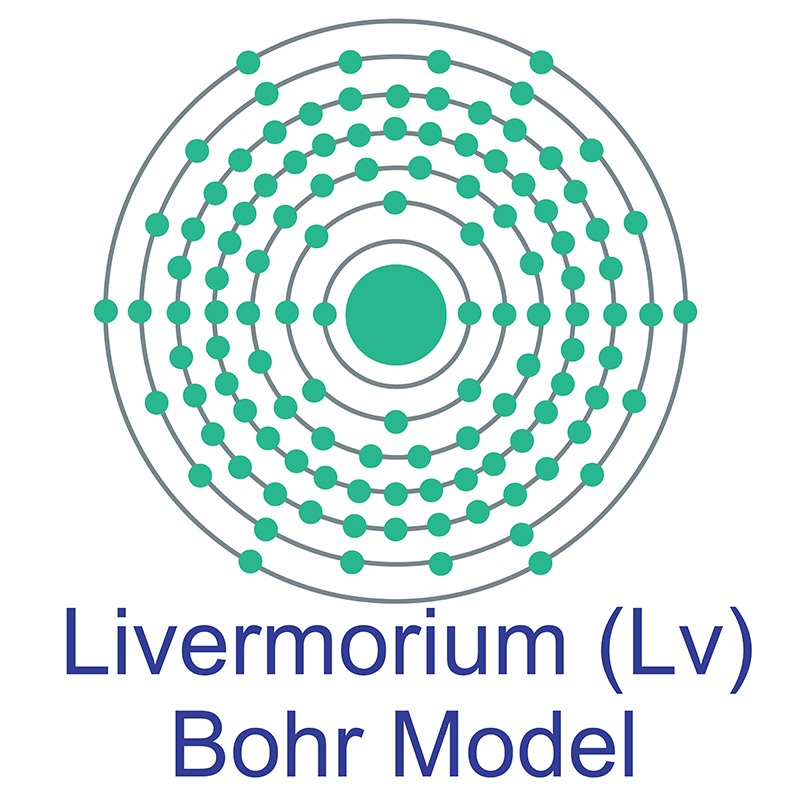 Livermorium is a P-Block, Group 16, Period 7 element. The number of electrons in each of Livermorium's shells is 2, 8, 18, 32, 32, 18, 6 and its electron configuration is [Rn] 5f14 6d10 7s27p4. In its elemental form Livermorium's CAS number is 54100-71-9. Livermorium was discovered in 2000 by the Joint Institute for Nuclear Research in Dubna, Russia and the Lawrence Livermore National Laboratory in Livermore, California, USA. Livermorium is a synthetic element that is not present in the environment. Little is known about the element, its appearance is unknown, and it has no known uses.
Livermorium is a P-Block, Group 16, Period 7 element. The number of electrons in each of Livermorium's shells is 2, 8, 18, 32, 32, 18, 6 and its electron configuration is [Rn] 5f14 6d10 7s27p4. In its elemental form Livermorium's CAS number is 54100-71-9. Livermorium was discovered in 2000 by the Joint Institute for Nuclear Research in Dubna, Russia and the Lawrence Livermore National Laboratory in Livermore, California, USA. Livermorium is a synthetic element that is not present in the environment. Little is known about the element, its appearance is unknown, and it has no known uses.
Livermorium information, including properties, research, applications and other useful facts are discussed below. Scientific facts such as the atomic structure, ionization energy, abundance on Earth, conductivity and thermal properties are included.
| Symbol: |
Lv |
| Atomic Number: |
116 |
| Atomic Weight: |
293 |
| Element Category: |
unknown |
| Group, Period, Block: |
16 (chalcogens), 7, p |
| Color: |
unknown (presumably metallic/ silvery white/ gray) |
| Other Names: |
Ununhexio |
| Melting Point: |
N/A |
| Boiling Point: |
N/A |
| Density: |
12.9 g·cm3 (predicted) |
| Liquid Density @ Melting Point: |
N/A |
| Density @ 20°C: |
N/A |
| Density of Solid: |
11200 (predicted) kg·m3 |
| Specific Heat: |
N/A |
| Superconductivity Temperature: |
N/A |
| Triple Point: |
N/A |
| Critical Point: |
N/A |
| Heat of Fusion (kJ·mol-1): |
N/A |
| Heat of Vaporization (kJ·mol-1): |
N/A |
| Heat of Atomization (kJ·mol-1): |
N/A |
| Thermal Conductivity: |
N/A |
| Thermal Expansion: |
N/A |
| Electrical Resistivity: |
N/A |
| Tensile Strength: |
N/A |
| Molar Heat Capacity: |
N/A |
| Young's Modulus: |
N/A |
| Shear Modulus: |
N/A |
| Bulk Modulus: |
N/A |
| Poisson Ratio: |
N/A |
| Mohs Hardness: |
N/A |
| Vickers Hardness: |
N/A |
| Brinell Hardness: |
N/A |
| Speed of Sound: |
N/A |
| Pauling Electronegativity: |
N/A |
| Sanderson Electronegativity: |
N/A |
| Allred Rochow Electronegativity: |
N/A |
| Mulliken-Jaffe Electronegativity: |
N/A |
| Allen Electronegativity: |
N/A |
| Pauling Electropositivity: |
N/A |
| Reflectivity (%): |
N/A |
| Refractive Index: |
N/A |
| Electrons: |
116 |
| Protons: |
116 |
| Neutrons: |
177 |
| Electron Configuration: |
[Rn] 5f14 6d10 7s27p4 |
| Atomic Radius: |
N/A |
Atomic Radius,
non-bonded (Å): |
Unknown |
| Covalent Radius: |
175 pm (estimated) |
| Covalent Radius (Å): |
1.75 |
| Van der Waals Radius: |
N/A |
| Oxidation States: |
2,4 (predicted) |
| Phase: |
Solid (predicted) |
| Crystal Structure: |
unknown |
| Magnetic Ordering: |
unknown |
| Electron Affinity (kJ·mol-1) |
Unknown |
| 1st Ionization Energy: |
723.6 kJ·mol-1(estimated) |
| 2nd Ionization Energy: |
N/A |
| 3rd Ionization Energy: |
N/A |
| CAS Number: |
54100-71-9 |
| EC Number: |
N/A |
| MDL Number: |
N/A |
| Beilstein Number: |
N/A |
| SMILES Identifier: |
N/A |
| InChI Identifier: |
N/A |
| InChI Key: |
N/A |
| PubChem CID: |
N/A |
| ChemSpider ID: |
N/A |
| Earth - Total: |
N/A |
| Mercury - Total: |
N/A |
| Venus - Total: |
N/A |
| Earth - Seawater (Oceans), ppb by weight: |
N/A |
| Earth - Seawater (Oceans), ppb by atoms: |
N/A |
| Earth - Crust (Crustal Rocks), ppb by weight: |
N/A |
| Earth - Crust (Crustal Rocks), ppb by atoms: |
N/A |
| Sun - Total, ppb by weight: |
N/A |
| Sun - Total, ppb by atoms: |
N/A |
| Stream, ppb by weight: |
N/A |
| Stream, ppb by atoms: |
N/A |
| Meterorite (Carbonaceous), ppb by weight: |
N/A |
| Meterorite (Carbonaceous), ppb by atoms: |
N/A |
| Typical Human Body, ppb by weight: |
N/A |
| Typical Human Body, ppb by atom: |
N/A |
| Universe, ppb by weight: |
N/A |
| Universe, ppb by atom: |
N/A |
| Discovered By: |
Joint Institute for Nuclear Research and Lawrence Livermore National Laboratory |
| Discovery Date: |
2000 |
| First Isolation: |
Henri Moissan (June 26, 1886) |
Livermorium Isotopes
Livermorium is an artificial element. Like all artificial elements, it has no stable isotopes.
| Nuclide |
Isotopic Mass |
Half-Life |
Mode of Decay |
Nuclear Spin |
Magnetic Moment |
Binding Energy (MeV) |
Natural Abundance
(% by atom) |
| 289Lv | 289.19886(117)# | 10# ms | Unknown | 5/2+# | N/A | 2027.58 | - |
| 291Lv | 290.19859(91)# | 15(+26-6) ms | a to 286Fl | 0+ | N/A | 2035.66 | - |
| 290Lv | 291.20001(91)# | 6.3(+116-25) ms | a to 287Fl | N/A | N/A | 2034.42 | - |
| 292Lv | 292.19979(92)# | 18.0(+16-6) ms | a to 288Fl | 0+ | N/A | 2051.81 | - |
| 293Lv | 293.20449(60)# | 61(+57-20) ms | a to 289Fl | N/A | N/A | N/A | - |

 Livermorium is a P-Block, Group 16, Period 7 element. The number of electrons in each of Livermorium's shells is 2, 8, 18, 32, 32, 18, 6 and its electron configuration is [Rn] 5f14 6d10 7s27p4. In its elemental form Livermorium's CAS number is 54100-71-9. Livermorium was discovered in 2000 by the Joint Institute for Nuclear Research in Dubna, Russia and the Lawrence Livermore National Laboratory in Livermore, California, USA. Livermorium is a synthetic element that is not present in the environment. Little is known about the element, its appearance is unknown, and it has no known uses.
Livermorium is a P-Block, Group 16, Period 7 element. The number of electrons in each of Livermorium's shells is 2, 8, 18, 32, 32, 18, 6 and its electron configuration is [Rn] 5f14 6d10 7s27p4. In its elemental form Livermorium's CAS number is 54100-71-9. Livermorium was discovered in 2000 by the Joint Institute for Nuclear Research in Dubna, Russia and the Lawrence Livermore National Laboratory in Livermore, California, USA. Livermorium is a synthetic element that is not present in the environment. Little is known about the element, its appearance is unknown, and it has no known uses.