SECTION 1. IDENTIFICATION
Product Name: (5N) 99.999% Germanium(IV) Sulfide
Product Number: All applicable American Elements product codes, e.g. GE4-S-05-P
CAS #: 12025-34-2
Relevant identified uses of the substance: Scientific research and development
Supplier details:
American Elements
10884 Weyburn Ave.
Los Angeles, CA 90024
Tel: +1 310-208-0551
Fax: +1 310-208-0351
Emergency telephone number:
Domestic, North America: +1 800-424-9300
International: +1 703-527-3887
SECTION 2. HAZARDS IDENTIFICATION
Emergency Overview
OSHA Hazards
No known OSHA hazards
HMIS Classification

Health Hazard: 2
Flammability: 0
Physical hazards: 0
NFPA Rating
Health Hazard: 2
Fire: 0
Reactivity Hazard: 0
Potential Health Effects
Eye Contact: May cause irritation or conjunctivitis by dissolving to form germanic acid, a weak acid.
Skin contact: May cause contact dermatitis.
Inhalation: Inhalation of particulates of will irritate the respiratory tract. Overexposure may produce coughing, headache and nausea.
Oral Toxicity: No data available
Chronic Toxicity: No specific information has been reported for this compound. Inhalation- Animal
studies of germanium compounds indicate morphological changes of the respiratory tract such as thickening of the alveolar partitions and hyperplasia of the lymphatic vessels around the bronchi and blood vessels. Ingestion- Rats fed 100ppm for 14 weeks showed stimulated growth. Those fed 1000ppm showed depressed growth and suffered 50% mortality. Kidney damage has been
reported in humans and animals. For selenium compounds, pallor, nervousness, depression and death have been reported for chronic exposure.
SECTION 3. COMPOSITION/INFORMATION ON INGREDIENTS
CAS Number: 12025-34-2
Formula : GeS2
Material: Germanium(IV) Sulfide
SECTION 4. FIRST AID MEASURES
EYES: In case of contact, immediately flush eyes with flowing water for at least 15 minutes. Get medical attention.
SKIN: Flush with water, then wash with soap and water.
INHALATION: Move exposed individual to fresh air. Call a physician.
INGESTION: Never give fluids or induce vomiting if patient is unconscious or having
convulsions. Get medical attention.
SECTION 5. FIREFIGHTING MEASURES
Flash Point, COC: Not Flammable Autoignition Temp.: not ignitable
Flammability Limits- LEL: NA UEL: NA
Extinguishing Media: N/A
Special Fire Fighting Procedures: Avoid eye and skin contact. Do not breathe fumes or inhale vapors.
Unusual Fire and Explosion Hazards: Irritating fumes vapors may develop when material is mixed with other materials and exposed to elevated temperatures or open flame.
SECTION 6. ACCIDENTAL RELEASE MEASURES
Personal precautions
Avoid dust formation.
Environmental precautions
Do not let product enter drains.
Methods for cleaning up
Sweep up and shovel. Keep in suitable, closed containers for disposal.
SECTION 7. HANDLING AND STORAGE
Handling
Provide appropriate exhaust ventilation at places where dust is formed.
Storage
Keep container tightly closed in a dry and well-ventilated place.
SECTION 8. EXPOSURE CONTROLS/PERSONAL PROTECTION
ENGINEERING CONTROLS
Safety shower and eye bath.
Mechanical exhaust required.
PERSONAL PROTECTIVE EQUIPMENT
Respiratory: Use respirators and components tested and approved under appropriate government
standards such as NIOSH (US) or CEN (EU). Respiratory protection is not required. Where
protection from nuisance levels of dusts are desired, use type N95
(US) or type P1 (EN 143) dust masks.
Hand: Protective gloves.
Eye: Chemical safety goggles.
GENERAL HYGIENE MEASURES
Wash thoroughly after handling.
SECTION 9. PHYSICAL AND CHEMICAL PROPERTIES
Boiling Point: NA
Melting Point: ca 800°C
Specific Gravity: 2.94
Vapor Pressure: No data available
Vapor Density: NA
Solubility in water: insoluble - reacts slowly
% volatiles: 0
Evaporation rate: NA
Molecular Weight: 136.73
Other: NA
Appearance & Color: Tan powder
SECTION 10. STABILITY AND REACTIVITY
Stability: Stable At temperatures >400°C in air oxidizes to germanium dioxide, sulfur and oxides of sulfur.
Incompatibility (materials to avoid): Compatible with most materials Dissolves in strong acid or base.
Hazardous decomposition products: Sulfides, germanium oxides.
SECTION 11. TOXICOLOGICAL INFORMATION
Acute toxicity
no data available
Irritation and corrosion
no data available
Sensitisation
no data available
Chronic exposure
IARC: No component of this product present at levels greater than or equal to 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.
ACGIH: No component of this product present at levels greater than or equal to 0.1% is identified as a carcinogen or potential carcinogen by ACGIH.
NTP: No component of this product present at levels greater than or equal to 0.1% is identified as a known or anticipated carcinogen by NTP.
OSHA: No component of this product present at levels greater than or equal to 0.1% is identified as a carcinogen or potential carcinogen by OSHA.
Potential Health Effects
Inhalation May be harmful if inhaled. May cause respiratory tract irritation.
Skin May be harmful if absorbed through skin. May cause skin irritation.
Eyes May cause eye irritation.
Ingestion May be harmful if swallowed.
SECTION 12. ECOLOGICAL INFORMATION
Spill response: May be hazardous to aquatic life if released to open waters. Sweep material while wearing a dust mask. Transfer to a suitable container for disposal.
Recommended Disposal: Dispose of as solid waste. Follow all chemical pollution control regulations. Large quantities may be recycled by return to the Gelest, the manufacturer.
SECTION 13. DISPOSAL CONSIDERATIONS
Product
Observe all federal, state, and local environmental regulations.
Contaminated packaging
Dispose of as unused product.
SECTION 14. TRANSPORT INFORMATION
DOT (US)
Not dangerous goods
IMDG
Not dangerous goods
IATA
Not dangerous goods
SECTION 15. REGULATORY INFORMATION
OSHA Hazards
No known OSHA hazards
TSCA Status
On TSCA Inventory
DSL Status
This product contains the following components listed on the Canadian NDSL list. All other components are on the Canadian DSL list.
SARA 302 Components
SARA 302: No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.
SARA 313 Components
SARA 313: This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.
SARA 311/312 Hazards
No SARA Hazards
SECTION 16. OTHER INFORMATION
Safety Data Sheet according to Regulation (EC) No. 1907/2006 (REACH). The above information is believed to be correct but does not purport to be all inclusive and shall be used only as a guide. The information in this document is based on the present state of our knowledge and is applicable to the product with regard to appropriate safety precautions. It does not represent any guarantee of the properties of the product. American Elements shall not be held liable for any damage resulting from handling or from contact with the above product. See reverse side of invoice or packing slip for additional terms and conditions of sale. COPYRIGHT 1997-2022 AMERICAN ELEMENTS. LICENSED GRANTED TO MAKE UNLIMITED PAPER COPIES FOR INTERNAL USE ONLY.
 The number of electrons in each of germanium's shells is 2, 8, 18, 4 and its electron configuration is [Ar] 3d10 4s2 4p2. The germanium atom has a radius of 122.5 pm and a Van der Waals radius of 211 pm. Germanium was first discovered by Clemens Winkler in 1886. In its elemental form, germanium is a brittle grayish white semi-
The number of electrons in each of germanium's shells is 2, 8, 18, 4 and its electron configuration is [Ar] 3d10 4s2 4p2. The germanium atom has a radius of 122.5 pm and a Van der Waals radius of 211 pm. Germanium was first discovered by Clemens Winkler in 1886. In its elemental form, germanium is a brittle grayish white semi- It is commercially obtained from
It is commercially obtained from 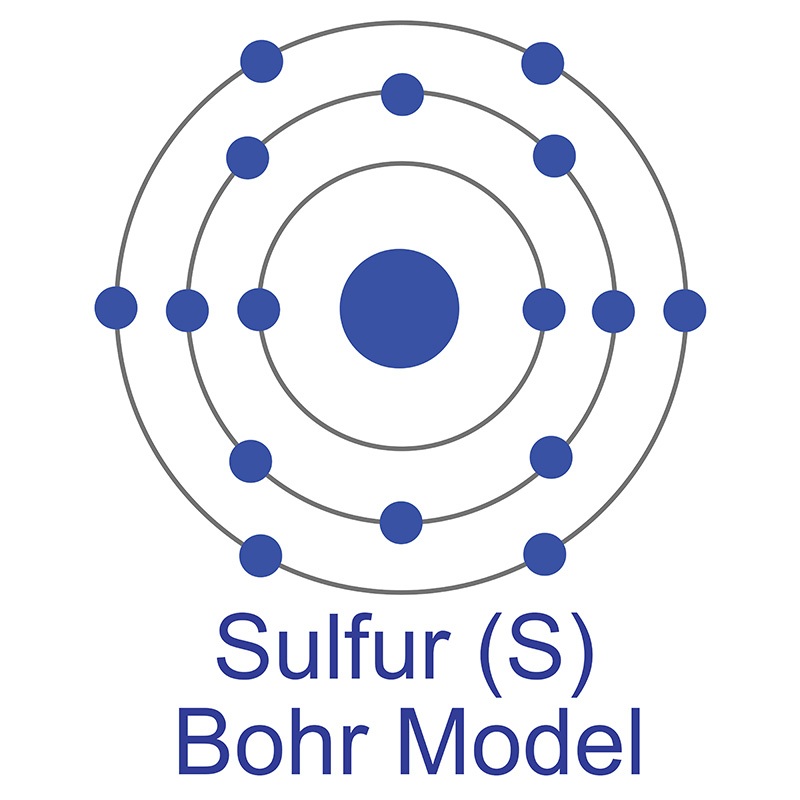 The number of electrons in each of Sulfur's shells is 2, 8, 6 and its electron configuration is [Ne] 3s2 3p4. In its elemental form, sulfur has a light yellow appearance. The sulfur atom has a covalent radius of 105 pm and a Van der Waals radius of 180 pm. In nature, sulfur can be found in hot springs, meteorites, volcanoes, and as galena, gypsum, and epsom salts. Sulfur has been known since ancient times but was not accepted as an element until 1777, when Antoine Lavoisier helped to convince the scientific community that it was an element and not a compound.
The number of electrons in each of Sulfur's shells is 2, 8, 6 and its electron configuration is [Ne] 3s2 3p4. In its elemental form, sulfur has a light yellow appearance. The sulfur atom has a covalent radius of 105 pm and a Van der Waals radius of 180 pm. In nature, sulfur can be found in hot springs, meteorites, volcanoes, and as galena, gypsum, and epsom salts. Sulfur has been known since ancient times but was not accepted as an element until 1777, when Antoine Lavoisier helped to convince the scientific community that it was an element and not a compound.