SECTION 1. IDENTIFICATION
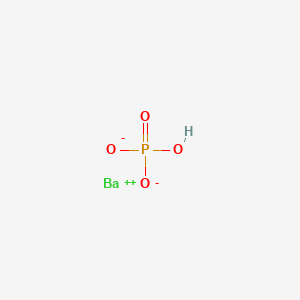
Product Name: Barium Phosphate Dibasic
Product Number: All applicable American Elements product codes, e.g. BA-PAT-01-P.DBASC
CAS #: 10048-98-3
Relevant identified uses of the substance: Scientific research and development
Supplier details:
American Elements
10884 Weyburn Ave.
Los Angeles, CA 90024
Tel: +1 310-208-0551
Fax: +1 310-208-0351
Emergency telephone number:
Domestic, North America: +1 800-424-9300
International: +1 703-527-3887
SECTION 2. HAZARDS IDENTIFICATION
CLASSIFICATION OF SUBSTANCE OR MIXTURE
Pictogram
Signal Word Warning
Hazard Statements
H302 Harmful if swallowed.
H332 Harmful if inhaled.
Precautionary Phrases
P261 Avoid breathing dust/fume/gas/mist/vapors/spray.
P264 Wash skin thoroughly after handling.
P304+P340 IF INHALED: Remove person to fresh air and keep comfortable for breathing.
P301+P312 IF SWALLOWED: Call a POISON CENTER/doctor if you feel unwell.
HMIS CLASSIFICATION:
Health: 2 Chronic: * Fire: 0 Reactivity Hazard: 0
NFPA RATING:
Health: 2 Flammability: 0 Reactivity Hazard: 0
EYE CONTACT: May be a mild irritant to the eyes.
SKIN CONTACT: May cause slight irritation of the skin.
INHALATION: Inhalation of dust can lead to irritation of the respiratory tract.
INGESTION: May be irritating if inhaled.
SECTION 3. COMPOSITION/INFORMATION ON INGREDIENTS
Formula: BaHPO4
Molecular Weight: 233.31
CHEMICAL NAME CAS# %
Barium Hydrogen Phosphate 10048-98-3 100
SECTION 4. FIRST AID MEASURES
EYE EXPOSURE: Immediately flush the eyes with copious amounts of water for at least 15 minutes. Assure flushing under
eyelids. A victim may need assistance in keeping their eyelids open. Get immediate competent medical attention.
SKIN EXPOSURE: Wash affected area with water. Remove contaminated clothes if necessary. Seek medical assistance if
irritation persists.
INHALATION: Remove to fresh air and keep at rest. Closely monitor the victim for signs of respiratory problems, such as
difficulty in breathing, coughing, wheezing, or pain. In such cases, seek immediate medical assistance.
INGESTION: Seek medical assistance immediately. Keep the victim calm. Give the victim water (only if conscious). Induce
vomiting only if directed by medical personnel.
SECTION 5. FIREFIGHTING MEASURES
FLASH POINT: Product is not flammable
Autoignition: Not available
EXPLOSION LIMITS: Not available
EXTINGUISHING MEDIUM: Use fire fighting measures that suit the surrounding fire.
SPECIAL FIRE FIGHTING PROCEDURES: Wear self-contained, approved breathing apparatus and full protective clothing,
including eye protection and boots.
HAZARDOUS COMBUSTION AND DECOMPOSITION PRODUCTS: Phosphorus oxides and barium oxide
SECTION 6. ACCIDENTAL RELEASE MEASURES
PERSONAL PRECAUTIONS: Wear all appropriate equipment when using this material. Ensure adequate ventilation. Avoid the
formation of dust. Avoid breathing vapors, mist, gas, or dust.
ENVIRONMENTAL PRECAUTIONS: Prevent spillage from entering drains or allowing to be released into the environment.
METHODS AND MATERIALS FOR CONTAINMENT AND CLEANING UP: Pick up and arrange disposal without creating dust. Sweep
up and keep in suitable, closed containers for disposal.
SECTION 7. HANDLING AND STORAGE
PRECAUTIONS FOR SAFE HANDLING: Wear appropriate personal protective equipment. Use with adequate ventilation. Avoid
contact with skin and eyes. Avoid formation of dust.
CONDITIONS FOR SAFE STORAGE: Store in cool, dry, and well-ventilated area. Store in a tightly sealed container.
SECTION 8. EXPOSURE CONTROLS/PERSONAL PROTECTION
EXPOSURE CONTROLS:
Component Exposure Limits Basis Entity
Barium Hydrogen Phosphate 0.5 mg/m3 TWA OSHA
0.5 mg/m3 TWA ACGIH
TWA: Time Weighted Average over 8 hours of work.
EYE PROTECTION: Wear chemical safety glasses or goggles and face shield.
SKIN PROTECTION: Wear nitrile or rubber gloves, apron, or lab coat.
VENTILATION: Provide local exhaust, preferably mechanical.
RESPIRATOR: Use an approved respirator.
ADDITIONAL PROTECTION: Provide eyewash stations, quick-drench showers and washing facilities accessible to areas of use and
handling.
SECTION 9. PHYSICAL AND CHEMICAL PROPERTIES
COLOR AND FORM: White powder
ODOR: None
MOLECULAR WEIGHT: 233.31
BOILING POINT: Decomposes
MELTING POINT: 410°C
SPECIFIC GRAVITY: 4.165 gm/ml
VAPOR DENSITY: No data available
SOLUBILITY: Slightly soluble
SECTION 10. STABILITY AND REACTIVITY
STABILITY: Stable
HAZARDOUS POLYMERIZATION: Will not occur
CONDITIONS TO AVOID: None.
INCOMPATIBILITY: Strong oxidizing agents
DECOMPOSITION PRODUCTS: Phosphorus oxides and barium oxide
SECTION 11. TOXICOLOGICAL INFORMATION
ACUTE TOXICITY: Not available
CARCINOGENIC EFFECTS: No components of this product present at levels greater than or equal to 0.1% is identified a carcinogen.
MUTAGENIC EFFECTS: Not available
TETRATOGENIC EFFECTS: Not available
RTECS: Not available
To the best of our knowledge the toxicological effects of this compound have not been fully investigated.
SECTION 12. ECOLOGICAL INFORMATION
No data available
SECTION 13. DISPOSAL CONSIDERATIONS
Dispose of in according to local, state, and federal regulations.
SECTION 14. TRANSPORT INFORMATION
DOT : UN1564 IATA: UN1564
Barium compounds, n.o.s. Barium compound, n.o.s.
(Barium Hydrogen Phosphate) (Barium Hydrogen Phosphate)
CLASS 6.1 CLASS 6.1
PG III PG III
Marine Pollutant: No
SECTION 15. REGULATORY INFORMATION
SARA 302/304: Not listed
SARA 311/312: Acute Health Hazard, Chronic Health Hazard
SARA (TITLE 313): Barium Hydrogen Phosphate
PENNSYLVANIA RIGHT TO KNOW: Barium Hydrogen Phosphate
NEW JERSEY RIGHT TO KNOW: Barium Hydrogen Phosphate
CALIFORNIA PROP. 65: Not Listed
SECTION 16. OTHER INFORMATION
Safety Data Sheet according to Regulation (EC) No. 1907/2006 (REACH). The above information is believed to be correct but does not purport to be all inclusive and shall be used only as a guide. The information in this document is based on the present state of our knowledge and is applicable to the product with regard to appropriate safety precautions. It does not represent any guarantee of the properties of the product. American Elements shall not be held liable for any damage resulting from handling or from contact with the above product. See reverse side of invoice or packing slip for additional terms and conditions of sale. COPYRIGHT 1997-2022 AMERICAN ELEMENTS. LICENSED GRANTED TO MAKE UNLIMITED PAPER COPIES FOR INTERNAL USE ONLY.

 Barium is a member of the alkaline-earth
Barium is a member of the alkaline-earth  In its elemental form, barium is a soft, silvery-gray metal. Industrial applications for barium include acting as a "getter," or unwanted gas remover, for vacuum tubes, and as an additive to steel and cast
In its elemental form, barium is a soft, silvery-gray metal. Industrial applications for barium include acting as a "getter," or unwanted gas remover, for vacuum tubes, and as an additive to steel and cast  See more Phosphorus products.
See more Phosphorus products.