SECTION 1. IDENTIFICATION
Product Name: Cobalt Monoantimonide
Product Number: All applicable American Elements product codes, e.g. CO-SB-02
, CO-SB-03
, CO-SB-04
, CO-SB-05
CAS #: 12052-42-5
Relevant identified uses of the substance: Scientific research and development
Supplier details:
American Elements
10884 Weyburn Ave.
Los Angeles, CA 90024
Tel: +1 310-208-0551
Fax: +1 310-208-0351
Emergency telephone number:
Domestic, North America: +1 800-424-9300
International: +1 703-527-3887
SECTION 2. HAZARDS IDENTIFICATION
2.1 Classification of the substance or mixture
2.2 GHS Label elements, including precautionary statements
2.3 Hazards not otherwise classified (HNOC) or not covered by GHS - none
SECTION 3. COMPOSITION/INFORMATION ON INGREDIENTS
3.1 Substances
Synonyms : Cobalt antimonide
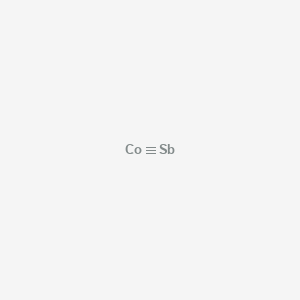
Formula : CoSb
Molecular weight : 180.69 g/mol
CAS-No. : 12052-42-5
EC-No. : 234-993-9
Index-No. : 051-003-00-9
Hazardous components
Component Classification Concentration
Cobalt monoantimonide
<= 100 %
SECTION 4. FIRST AID MEASURES
4.1 Description of first aid measures
General advice
Consult a physician. Show this safety data sheet to the doctor in attendance.Move out of dangerous area.
If inhaled
If breathed in, move person into fresh air. If not breathing, give artificial respiration. Consult a physician.
In case of skin contact
Wash off with soap and plenty of water. Take victim immediately to hospital. Consult a physician.
In case of eye contact
Rinse thoroughly with plenty of water for at least 15 minutes and consult a physician.
If swallowed
Never give anything by mouth to an unconscious person. Rinse mouth with water. Consult a physician.
4.2 Most important symptoms and effects, both acute and delayed
The most important known symptoms and effects are described in the labelling (see section 2.2) and/or in section 11
4.3 Indication of any immediate medical attention and special treatment needed
No data available
SECTION 5. FIREFIGHTING MEASURES
5.1 Extinguishing media
Suitable extinguishing media
Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide.
5.2 Special hazards arising from the substance or mixture
Cobalt/cobalt oxides, Antimony oxide
5.3 Advice for firefighters
Wear self-contained breathing apparatus for firefighting if necessary.
5.4 Further information
No data available
SECTION 6. ACCIDENTAL RELEASE MEASURES
6.1 Personal precautions, protective equipment and emergency procedures
Wear respiratory protection. Avoid dust formation. Avoid breathing Vapors, mist or gas. Ensure adequate ventilation.
Evacuate personnel to safe areas. Avoid breathing dust.
For personal protection see section 8.
6.2 Environmental precautions
Prevent further leakage or spillage if safe to do so. Do not let product enter drains. Discharge into the environment
must be avoided.
6.3 Methods and materials for containment and cleaning up
Pick up and arrange disposal without creating dust. Sweep up and shovel. Keep in suitable, closed containers for
disposal.
6.4 Reference to other sections
For disposal see section 13.
SECTION 7. HANDLING AND STORAGE
7.1 Precautions for safe handling
Avoid contact with skin and eyes. Avoid formation of dust and aerosols.
Provide appropriate exhaust ventilation at places where dust is formed.
For precautions see section 2.2.
7.2 Conditions for safe storage, including any incompatibilities
Keep container tightly closed in a dry and well-ventilated place.
7.3 Specific end use(s)
Apart from the uses mentioned in section 1.2 no other specific uses are stipulated
SECTION 8. EXPOSURE CONTROLS/PERSONAL PROTECTION
8.1 Control parameters
Components with workplace control parameters
Component CAS-No. Value Control
parameters
Basis
Cobalt
monoantimonide
12052-42-5 TWA 0.500000
mg/m3
USA. Occupational Exposure Limits
(OSHA) - Table Z-1 Limits for Air
Contaminants
TWA 0.500000
mg/m3
USA. ACGIH Threshold Limit Values
(TLV)
Remarks Upper Respiratory Tract irritation
Skin irritation
TWA 0.020000
mg/m3
USA. ACGIH Threshold Limit Values
(TLV)
Pulmonary function
Asthma
Myocardial effects
Substances for which there is a Biological Exposure Index or Indices
(see BEI® section)
Confirmed animal carcinogen with unknown relevance to humans
varies
TWA 0.500000
mg/m3
USA. NIOSH Recommended
Exposure Limits
8.2 Exposure controls
Appropriate engineering controls
Avoid contact with skin, eyes and clothing. Wash hands before breaks and immediately after handling the product.
Personal protective equipment
Eye/face protection
Face shield and safety glasses Use equipment for eye protection tested and approved under appropriate
government standards such as NIOSH (US) or EN 166(EU).
Skin protection
Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique (without
touching glove's outer surface) to avoid skin contact with this product. Dispose of contaminated gloves after
use in accordance with applicable laws and good laboratory practices. Wash and dry hands.
Body Protection
Complete suit protecting against chemicals, The type of protective equipment must be selected according to
the concentration and amount of the dangerous substance at the specific workplace.
Respiratory protection
Where risk assessment shows air-purifying respirators are appropriate use a full-face particle respirator type
N99 (US) or type P2 (EN 143) respirator cartridges as a backup to engineering controls. If the respirator is the
sole means of protection, use a full-face supplied air respirator. Use respirators and components tested and
approved under appropriate government standards such as NIOSH (US) or CEN (EU).
Control of environmental exposure
Prevent further leakage or spillage if safe to do so. Do not let product enter drains. Discharge into the
environment must be avoided.
SECTION 9. PHYSICAL AND CHEMICAL PROPERTIES
9.1 Information on basic physical and chemical properties
a) Appearance Form: solid
b) Odor No data available
c) Odor Threshold No data available
d) pH No data available
e) Melting point/freezing
point
Melting point/range: 1,202 °C (2,196 °F) - lit.
f) Initial boiling point and
boiling range
No data available
g) Flash point No data available
h) Evaporation rate No data available
i) Flammability (solid, gas) No data available
j) Upper/lower
flammability or
explosive limits
No data available
k) Vapor pressure No data available
l) Vapor density No data available
m) Relative density No data available
n) Water solubility No data available
o) Partition coefficient: noctanol/
water
No data available
p) Auto-ignition
temperature
No data available
q) Decomposition
temperature
No data available
r) Viscosity No data available
s) Explosive properties No data available
t) Oxidizing properties No data available
9.2 Other safety information
No data available
SECTION 10. STABILITY AND REACTIVITY
10.1 Reactivity
No data available
10.2 Chemical stability
Stable under recommended storage conditions.
10.3 Possibility of hazardous reactions
No data available
10.4 Conditions to avoid
No data available
10.5 Incompatible materials
Strong acids
10.6 Hazardous decomposition products
Other decomposition products - No data available
In the event of fire: see section 5
SECTION 11. TOXICOLOGICAL INFORMATION
11.1 Information on toxicological effects
Acute toxicity
No data available
Inhalation: No data available
Dermal: No data available
No data available
Skin corrosion/irritation
No data available
Serious eye damage/eye irritation
No data available
Respiratory or skin sensitisation
No data available
Germ cell mutagenicity
No data available
Carcinogenicity
IARC: 2B - Group 2B: Possibly carcinogenic to humans (Cobalt monoantimonide)
NTP: No component of this product present at levels greater than or equal to 0.1% is identified as a
known or anticipated carcinogen by NTP.
OSHA: No component of this product present at levels greater than or equal to 0.1% is identified as a
carcinogen or potential carcinogen by OSHA.
Reproductive toxicity
No data available
No data available
Specific target organ toxicity - single exposure
Inhalation - May cause respiratory irritation.
Specific target organ toxicity - repeated exposure
No data available
Aspiration hazard
No data available
Additional Information
RTECS: Not available
dry throat, Cough, Lung irritation, Difficulty in breathing, Skin irritation, Eye irritation, May cause headache and
dizziness., Headache, May cause nausea, abdominal spasms and irritation of the mucous membranes., Fever, cramps,
Vomiting, Diarrhoea, Anorexia., metallic taste, Salivation, Dizziness, moderate to severe pain, sleep disturbances
Stomach - Irregularities - Based on Human Evidence
Stomach - Irregularities - Based on Human Evidence
SECTION 12. ECOLOGICAL INFORMATION
12.1 Toxicity
No data available
12.2 Persistence and degradability
No data available
12.3 Bioaccumulative potential
No data available
12.4 Mobility in soil
No data available
12.5 Results of PBT and vPvB assessment
PBT/vPvB assessment not available as chemical safety assessment not required/not conducted
12.6 Other adverse effects
Toxic to aquatic life with long lasting effects.
An environmental hazard cannot be excluded in the event of unprofessional handling or disposal.
No data available
SECTION 13. DISPOSAL CONSIDERATIONS
13.1 Waste treatment methods
Product
Offer surplus and non-recyclable solutions to a licensed disposal company. Contact a licensed professional waste
disposal service to dispose of this material. Dissolve or mix the material with a combustible solvent and burn in a
chemical incinerator equipped with an afterburner and scrubber.
Contaminated packaging
Dispose of as unused product.
SECTION 14. TRANSPORT INFORMATION
DOT (US)
UN number: 1549 Class: 6.1 Packing group: III
Proper shipping name: Antimony compounds, inorganic, solid, n.o.s. (Cobalt monoantimonide)
Reportable Quantity (RQ):
Poison Inhalation Hazard: No
IMDG
UN number: 1549 Class: 6.1 Packing group: III EMS-No: F-A, S-A
Proper shipping name: ANTIMONY COMPOUND, INORGANIC, SOLID, N.O.S. (Cobalt monoantimonide)
Marine pollutant:yes
IATA
UN number: 1549 Class: 6.1 Packing group: III
Proper shipping name: Antimony compound, inorganic, solid, n.o.s. (Cobalt monoantimonide)
SECTION 15. REGULATORY INFORMATION
SARA 302 Components
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.
SARA 313 Components
The following components are subject to reporting levels established by SARA Title III, Section 313:
Cobalt monoantimonide
CAS-No.
12052-42-5
Revision Date
2007-07-01
SARA 311/312 Hazards
Acute Health Hazard, Chronic Health Hazard
Massachusetts Right To Know Components
No components are subject to the Massachusetts Right to Know Act.
Pennsylvania Right To Know Components
Cobalt monoantimonide
CAS-No.
12052-42-5
Revision Date
2007-07-01
New Jersey Right To Know Components
Cobalt monoantimonide
CAS-No.
12052-42-5
Revision Date
2007-07-01
California Prop. 65 Components
This product does not contain any chemicals known to State of California to cause cancer, birth defects, or any other
reproductive harm.
SECTION 16. OTHER INFORMATION
Safety Data Sheet according to Regulation (EC) No. 1907/2006 (REACH). The above information is believed to be correct but does not purport to be all inclusive and shall be used only as a guide. The information in this document is based on the present state of our knowledge and is applicable to the product with regard to appropriate safety precautions. It does not represent any guarantee of the properties of the product. American Elements shall not be held liable for any damage resulting from handling or from contact with the above product. See reverse side of invoice or packing slip for additional terms and conditions of sale. COPYRIGHT 1997-2022 AMERICAN ELEMENTS. LICENSED GRANTED TO MAKE UNLIMITED PAPER COPIES FOR INTERNAL USE ONLY.
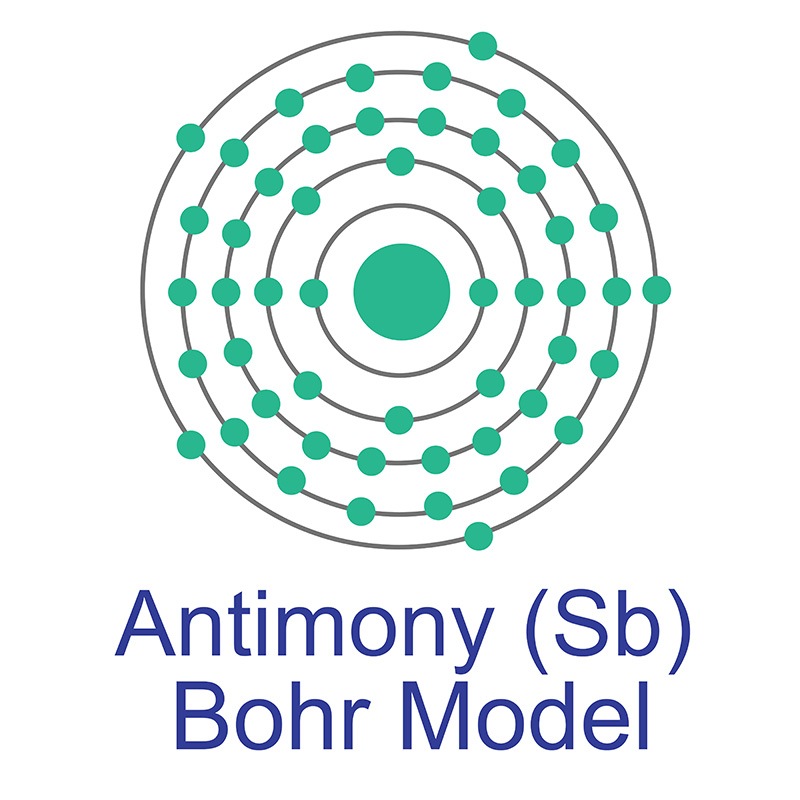 The number of electrons in each of antimony's shells is 2, 8, 18, 18, 5 and its electron configuration is [Kr] 4d10 5s2 5p3. The antimony atom has a radius of 140 pm and a Van der Waals radius of 206 pm. Antimony was discovered around 3000 BC and first isolated by Vannoccio Biringuccio in 1540 AD. In its elemental form, antimony has a silvery lustrous gray appearance.
The number of electrons in each of antimony's shells is 2, 8, 18, 18, 5 and its electron configuration is [Kr] 4d10 5s2 5p3. The antimony atom has a radius of 140 pm and a Van der Waals radius of 206 pm. Antimony was discovered around 3000 BC and first isolated by Vannoccio Biringuccio in 1540 AD. In its elemental form, antimony has a silvery lustrous gray appearance.  The most common source of antimony is the sulfide mineral known as stibnite (Sb2S3), although it sometimes occurs natively as well. Antimony has numerous applications, most commonly in flame-retardant materials. It also increases the hardness and strength of lead when combined in an alloy and is frequently employed as a dopant in semiconductor materials. Its name is derived from the Greek words anti and monos, meaning a metal not found by itself.
The most common source of antimony is the sulfide mineral known as stibnite (Sb2S3), although it sometimes occurs natively as well. Antimony has numerous applications, most commonly in flame-retardant materials. It also increases the hardness and strength of lead when combined in an alloy and is frequently employed as a dopant in semiconductor materials. Its name is derived from the Greek words anti and monos, meaning a metal not found by itself.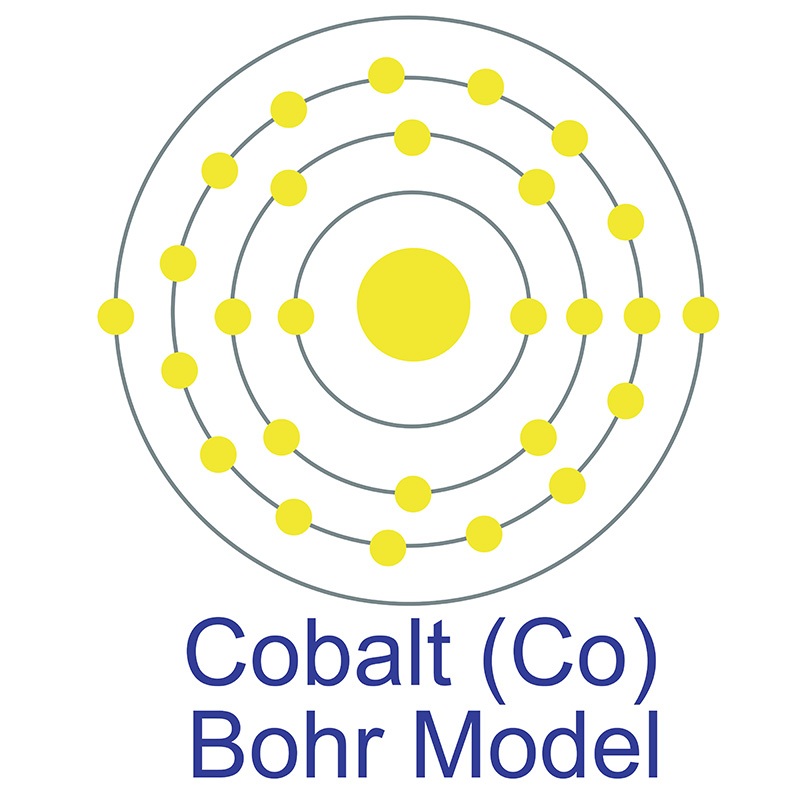 The number of electrons in each of cobalt's shells is 2, 8, 15, 2 and its electron configuration is [Ar]3d7 4s2. The cobalt atom has a radius of 125 pm and a Van der Waals radius of 192 pm. Cobalt was first discovered by George Brandt in 1732. In its elemental form, cobalt has a lustrous gray appearance. Cobalt is found in cobaltite, erythrite, glaucodot and skutterudite ores.
The number of electrons in each of cobalt's shells is 2, 8, 15, 2 and its electron configuration is [Ar]3d7 4s2. The cobalt atom has a radius of 125 pm and a Van der Waals radius of 192 pm. Cobalt was first discovered by George Brandt in 1732. In its elemental form, cobalt has a lustrous gray appearance. Cobalt is found in cobaltite, erythrite, glaucodot and skutterudite ores.  Cobalt produces brilliant blue pigments which have been used since ancient times to color paint and glass. Cobalt is a ferromagnetic metal and is used primarily in the production of
Cobalt produces brilliant blue pigments which have been used since ancient times to color paint and glass. Cobalt is a ferromagnetic metal and is used primarily in the production of