SECTION 1. IDENTIFICATION
Product Name: Ferric Sulfate
Product Number: All applicable American Elements product codes, e.g. FE3-SAT-02
, FE3-SAT-025
, FE3-SAT-03
, FE3-SAT-035
, FE3-SAT-04
, FE3-SAT-05
CAS #: 10028-22-5
Relevant identified uses of the substance: Scientific research and development
Supplier details:
American Elements
10884 Weyburn Ave.
Los Angeles, CA 90024
Tel: +1 310-208-0551
Fax: +1 310-208-0351
Emergency telephone number:
Domestic, North America: +1 800-424-9300
International: +1 703-527-3887
SECTION 2. HAZARDS IDENTIFICATION
Classification
This chemical is considered hazardous by the 2012 OSHA Hazard Communication Standard (29 CFR 1910.1200)
Acute oral toxicity Category 4
Skin Corrosion/Irritation Category 2
Serious Eye Damage/Eye Irritation Category 1


Signal Word
Danger
Hazard Statements
Harmful if swallowed
Causes skin irritation
Causes serious eye damage
Precautionary Statements
Prevention
Wash face, hands and any exposed skin thoroughly after handling
Do not eat, drink or smoke when using this product
Wear protective gloves/protective clothing/eye protection/face protection
Skin
IF ON SKIN: Wash with plenty of soap and water
If skin irritation occurs: Get medical advice/attention
Take off contaminated clothing and wash before reuse
Eyes
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing
Immediately call a POISON CENTER or doctor/physician
Ingestion
IF SWALLOWED: Call a POISON CENTER or doctor/physician if you feel unwell
Rinse mouth
Disposal
Dispose of contents/container to an approved waste disposal plant
Hazards not otherwise classified (HNOC)
None identified
SECTION 3. COMPOSITION/INFORMATION ON INGREDIENTS
Component
Ferric sulfate
CAS-No
10028-22-5
SECTION 4. FIRST AID MEASURES
General Advice If symptoms persist, call a physician.
Eye Contact Rinse immediately with plenty of water, also under the eyelids, for at least 15 minutes. Get medical attention.
Skin Contact Wash off immediately with plenty of water for at least 15 minutes. If skin irritation persists, call a physician.
Inhalation Remove to fresh air. If not breathing, give artificial respiration. Get medical attention if
symptoms occur.
Ingestion Clean mouth with water and drink afterwards plenty of water. Get medical attention if
symptoms occur.
Most important symptoms and effects
Causes eye burns. Causes severe eye damage.
Notes to Physician Treat symptomatically
SECTION 5. FIREFIGHTING MEASURES
Suitable Extinguishing Media Not combustible.
Unsuitable Extinguishing Media No information available
Flash Point No information available
Method - No information available
Autoignition Temperature No information available
Explosion Limits
Upper No data available
Lower No data available
Sensitivity to Mechanical Impact No information available
Sensitivity to Static Discharge No information available
Specific Hazards Arising from the Chemical
Keep product and empty container away from heat and sources of ignition.
Hazardous Combustion Products
Sulfur oxides. Iron oxides.
Protective Equipment and Precautions for Firefighters
As in any fire, wear self-contained breathing apparatus pressure-demand, MSHA/NIOSH (approved or equivalent) and full protective gear.
SECTION 6. ACCIDENTAL RELEASE MEASURES
Personal Precautions Ensure adequate ventilation. Use personal protective equipment as required. Avoid dust formation.
Environmental Precautions Should not be released into the environment. See Section 12 for additional Ecological
Information. Do not flush into surface water or sanitary sewer system. Do not allow material
to contaminate ground water system.
Methods for Containment and Clean Up
Sweep up and shovel into suitable containers for disposal. Keep in suitable, closed
containers for disposal.
SECTION 7. HANDLING AND STORAGE
Handling Wear personal protective equipment/face protection. Ensure adequate ventilation. Do not
get in eyes, on skin, or on clothing. Avoid ingestion and inhalation. Avoid dust formation.
Storage Store under an inert atmosphere. Keep container tightly closed in a dry and well-ventilated
place. Protect from moisture.
SECTION 8. EXPOSURE CONTROLS/PERSONAL PROTECTION
Eye/face Protection Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin and body protection Wear appropriate protective gloves and clothing to prevent skin exposure.
Respiratory Protection Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Hygiene Measures
Handle in accordance with good industrial hygiene and safety practice.
SECTION 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical State Solid
Appearance No information available
Odor Odorless
Odor Threshold No information available
pH No information available
Melting Point/Range 480 °C / 896 °F
Boiling Point/Range No information available
Flash Point No information available
Evaporation Rate Not applicable
Flammability (solid,gas) No information available
Flammability or explosive limits
Upper No data available
Lower No data available
Vapor Pressure No information available
Vapor Density Not applicable
Specific Gravity 3.097 g/cm3
Solubility No information available
Partition coefficient; n-octanol/water No data available
Autoignition Temperature No information available
Decomposition Temperature No information available
Viscosity Not applicable
Molecular Formula Fe2 (SO4)3
Molecular Weight 399.87
SECTION 10. STABILITY AND REACTIVITY
Reactive Hazard None known, based on information available
Stability Hygroscopic.
Conditions to Avoid Exposure to moist air or water.
Incompatible Materials Oxidizing agent
Hazardous Decomposition Products Sulfur oxides, Iron oxides
Hazardous Polymerization Hazardous polymerization does not occur.
Hazardous Reactions None under normal processing.
SECTION 11. TOXICOLOGICAL INFORMATION
Toxicologically Synergistic
Products
No information available
Delayed and immediate effects as well as chronic effects from short and long-term exposure
Irritation No information available
Sensitization No information available
Carcinogenicity The table below indicates whether each agency has listed any ingredient as a carcinogen.
Mutagenic Effects No information available
Reproductive Effects No information available.
Developmental Effects No information available.
Teratogenicity No information available.
STOT - single exposure None known
STOT - repeated exposure None known
Aspiration hazard No information available
Symptoms / effects,both acute and delayed
No information available
Endocrine Disruptor Information No information available
Other Adverse Effects The toxicological properties have not been fully investigated.
SECTION 12. ECOLOGICAL INFORMATION
Ecotoxicity
Contains a substance which is:. Harmful to aquatic organisms. May cause long-term adverse effects in the environment. Do not allow material to contaminate ground water
system.
Persistence and Degradability May persist
Bioaccumulation/ Accumulation No information available.
Mobility No information available.
SECTION 13. DISPOSAL CONSIDERATIONS
Waste Disposal Methods Chemical waste generators must determine whether a discarded chemical is classified as a hazardous waste. Chemical waste generators must also consult local, regional, and national hazardous waste regulations to ensure complete and accurate classification.
SECTION 14. TRANSPORT INFORMATION
DOT Not regulated
TDG Not regulated
IATA Not regulated
SECTION 15. REGULATORY INFORMATION
U.S. Federal Regulations
SARA 313 Not applicable
SARA 311/312 Hazard Categories See section 2 for more information
Clean Air Act Not applicable
OSHA - Occupational Safety and
Health Administration
Not applicable
CERCLA Not applicable
California Proposition 65 This product does not contain any Proposition 65 chemicals.
U.S. Department of Transportation
Reportable Quantity (RQ): N
DOT Marine Pollutant N
DOT Severe Marine Pollutant N
U.S. Department of Homeland Security
This product does not contain any DHS chemicals.
SECTION 16. OTHER INFORMATION
Safety Data Sheet according to Regulation (EC) No. 1907/2006 (REACH). The above information is believed to be correct but does not purport to be all inclusive and shall be used only as a guide. The information in this document is based on the present state of our knowledge and is applicable to the product with regard to appropriate safety precautions. It does not represent any guarantee of the properties of the product. American Elements shall not be held liable for any damage resulting from handling or from contact with the above product. See reverse side of invoice or packing slip for additional terms and conditions of sale. COPYRIGHT 1997-2022 AMERICAN ELEMENTS. LICENSED GRANTED TO MAKE UNLIMITED PAPER COPIES FOR INTERNAL USE ONLY.
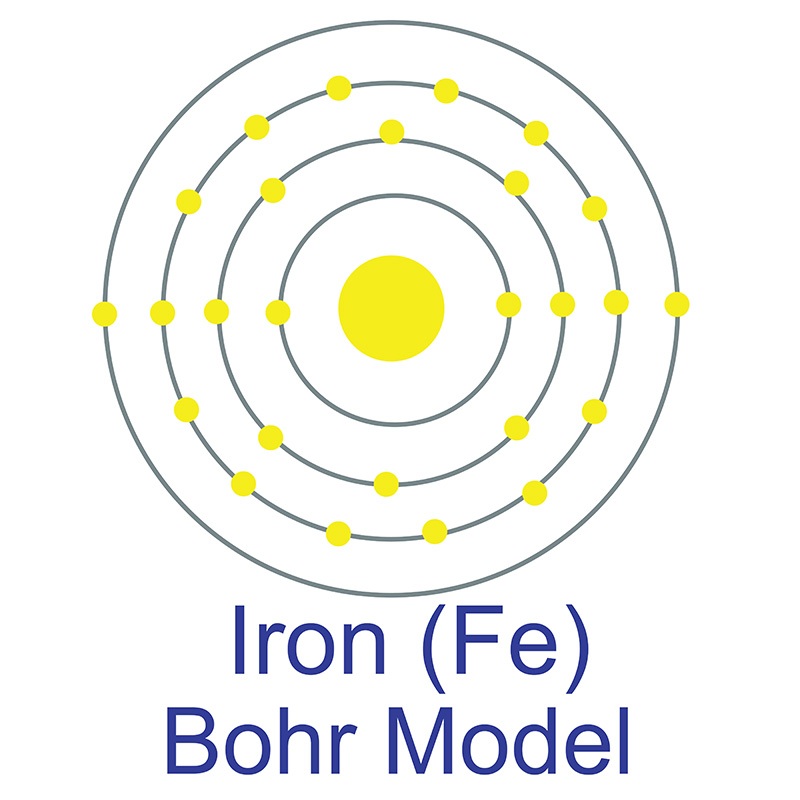 The iron atom has a radius of 126 pm and a Van der Waals radius of 194 pm. Iron was discovered by humans before 5000 BC. In its elemental form, iron has a lustrous grayish metallic appearance. Iron is the fourth most common element in the Earth's crust and the most common element by mass forming the earth as a whole. Iron is rarely found as a free element, since it tends to oxidize easily; it is usually found in minerals such as magnetite, hematite, goethite, limonite, or siderite.
The iron atom has a radius of 126 pm and a Van der Waals radius of 194 pm. Iron was discovered by humans before 5000 BC. In its elemental form, iron has a lustrous grayish metallic appearance. Iron is the fourth most common element in the Earth's crust and the most common element by mass forming the earth as a whole. Iron is rarely found as a free element, since it tends to oxidize easily; it is usually found in minerals such as magnetite, hematite, goethite, limonite, or siderite. Though pure iron is typically soft, the addition of carbon creates the
Though pure iron is typically soft, the addition of carbon creates the 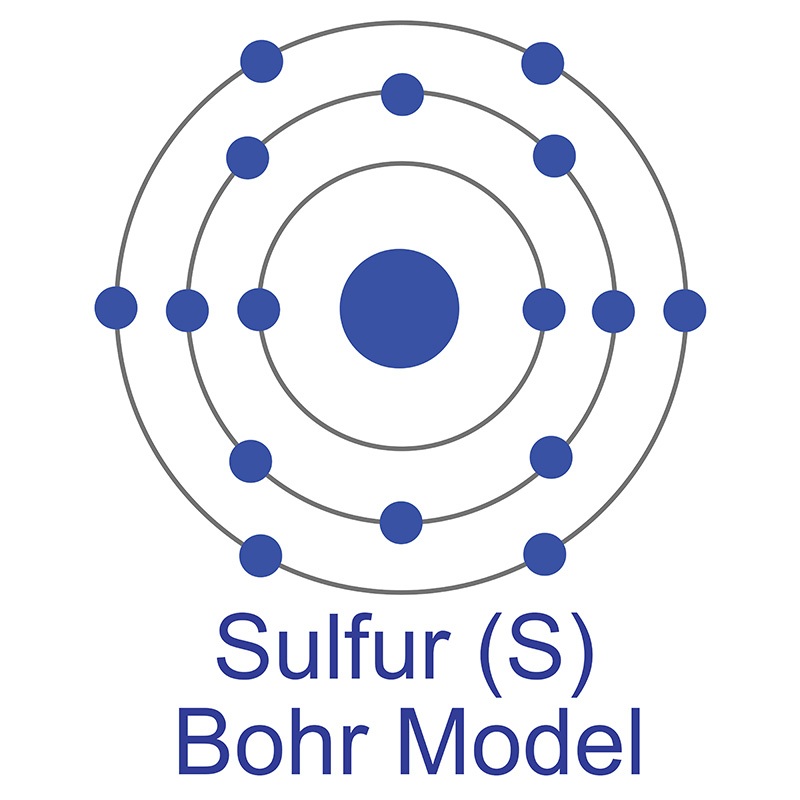 The number of electrons in each of Sulfur's shells is 2, 8, 6 and its electron configuration is [Ne] 3s2 3p4. In its elemental form, sulfur has a light yellow appearance. The sulfur atom has a covalent radius of 105 pm and a Van der Waals radius of 180 pm. In nature, sulfur can be found in hot springs, meteorites, volcanoes, and as galena, gypsum, and epsom salts. Sulfur has been known since ancient times but was not accepted as an element until 1777, when Antoine Lavoisier helped to convince the scientific community that it was an element and not a compound.
The number of electrons in each of Sulfur's shells is 2, 8, 6 and its electron configuration is [Ne] 3s2 3p4. In its elemental form, sulfur has a light yellow appearance. The sulfur atom has a covalent radius of 105 pm and a Van der Waals radius of 180 pm. In nature, sulfur can be found in hot springs, meteorites, volcanoes, and as galena, gypsum, and epsom salts. Sulfur has been known since ancient times but was not accepted as an element until 1777, when Antoine Lavoisier helped to convince the scientific community that it was an element and not a compound.