SECTION 1. IDENTIFICATION
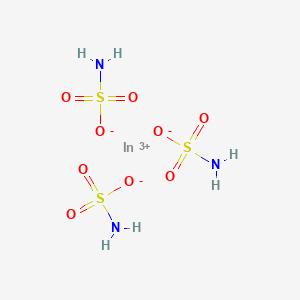
Product Name: Indium Sulfamate
Product Number: All applicable American Elements product codes, e.g. IN-SMAT-02-SOL
, IN-SMAT-03-SOL
, IN-SMAT-04-SOL
, IN-SMAT-01-C.AHYD
CAS #: 66027-93-8
Relevant identified uses of the substance: Scientific research and development
Supplier details:
American Elements
10884 Weyburn Ave.
Los Angeles, CA 90024
Tel: +1 310-208-0551
Fax: +1 310-208-0351
Emergency telephone number:
Domestic, North America: +1 800-424-9300
International: +1 703-527-3887
SECTION 2. HAZARDS IDENTIFICATION
PRIMARY ROUTES OF ENTRY:
Eye
Inhalation
Skin
Ingestion
CARCINOGEN LISTED IN:
NTP
IARC
OSHA
Not Listed


Signal Word: Warning
Hazard statement(s)
H303 May be harmful if swallowed
H315 Causes skin irritation
H319 Causes serious eye irritation
H333 May be harmful if inhaled
Precautionary statement(s)
P233 Keep container tightly closed
P261 Avoid breathing dust/fume/gas/mist/vapors/spray
P270 Do not eat, drink or smoke when using this product
P273 Avoid release to the environment
P280 Wear protective gloves/protective clothing/eye protection/face protection
P362 Take off contaminated clothing and wash before reuse
P301 + P314 IF SWALLOWED: Get Medical advice/attention if you feel unwell
P302 +P352 IF ON SKIN: Wash with plenty of soap and water
P304 + 341 IF INHALED: If breathing is difficult, remove victim to fresh air and keep at rest in a position comfortable for breathing
P305 + 351 F IN EYES: Rinse continuously with water for several minutes (15 mins)
POTENTIAL HEALTH EFFECTS:
Eye Contact: May cause severe irritation and possible burns.
Ingestion: May cause burning and damage of mouth, stomach and other internal organs.
Inhalation: Possible damage to nasal and respiratory passages and mucous membranes.
Skin Contact: May cause skin irritation or burns.
Chronic: Signs and symptoms of exposure are difficulty breathing, watery eyes, red irritating skin, abdominal pain and discomfort. Kidney and liver damage from injecting indium compounds has been reported based on limited animal testing but no systematic effects from human exposure has been reported.
SECTION 3. COMPOSITION/INFORMATION ON INGREDIENTS
Components
INDIUM SULFAMATE
CAS Registry #/EINECS
66027-93-8/266-072-2
SECTION 4. FIRST AID MEASURES
Eye Contact: Hold eyelids apart and flush eyes with plenty of water for at least 15 minutes. Seek medical attention if irritation persists.
Ingestion: If patient is conscious, ONLY induce vomiting as directed by trained personnel. NEVER give anything by mouth to an unconscious person. Seek medical attention immediately.
Inhalation: Remove to fresh air. If not breathing, give artificial respiration or oxygen by trained personnel. Seek immediate medical attention.
Skin Contact: Remove contaminated clothing. Wash affected area with soap and water. Wash clothing before reuse. If irritation persists, obtain medical attention.
SECTION 5. FIREFIGHTING MEASURES
Flash Point: Not established.
Method: Not established.
Auto-ignition Temperature: Not established.
Flammable Limits: Limits not established. Not flammable. Will not burn.
Extinguishing Media: Use extinguishers appropriate for the surrounding fire conditions.
Special Fire Fighting Procedures: Firefighters must wear NIOSH approved self-contained breathing apparatus and full protective clothing.
SECTION 6. ACCIDENTAL RELEASE MEASURES
Spill or Leak Procedures: Isolate spill and clean up with absorbent or neutralize. Place material in proper waste container. An approved air purifying respirator with acid gas/particulate cartridge and other personal protective equipment such as gloves is recommended. Dispose of in accordance with Federal, State and Local regulations. In Europe dispose of following the Special Waste Regulations. Indium may have reclaim value.
SECTION 7. HANDLING AND STORAGE
Handling: Keep containers tightly closed when not in use. Use care to avoid spills. Wear appropriate personal protective equipment. Use good work practices when handling corrosive material. Always thoroughly wash your hands after handling this product. DO NOT touch or rub eyes until hands are washed.
Storage Precautions: Store product in tightly capped original containers in a cool, well ventilated and dry area
SECTION 8. EXPOSURE CONTROLS/PERSONAL PROTECTION
Engineering Controls: Exhaust ventilation is recommended to control any air contaminants.
Personal protection:
Eyes: Wear chemical safety glasses/goggles. The addition of a face shield may be necessary during some work practices.
Respirator: A NIOSH approved air-purifying respirator with an acid gas/particulate filter is recommended under certain circumstances where airborne concentrations are expected to be elevated.
Skin: Compatible chemical resistant gloves. Rubber or vinyl gloves are recommended.
Other: Lab coat, apron, eyewash fountain in work area. Avoid the use of contact lenses in high fume/splash areas.
Work/Hygienic Maintain good housekeeping. Clean up spills immediately. Good personal hygiene is essential. Avoid eating, smoking or drinking in the work area. Wash hands thoroughly with soap and water immediately upon leaving the work area.
SECTION 9. PHYSICAL AND CHEMICAL PROPERTIES
Appearance: Clear or amber liquid
Odor: Odorless
Specific Gravity: H2O = 1
Vapor Pressure: N/A.
Vapor Density:N/A.
Boiling Point: Not established
Melting Point: N/A
pH: 2 (Corrosive)
Solubility in Water: Soluble
Note: when using product the pH is adjusted as needed. Review the Product Data Sheet for proper use of the plating bath solution. Preferred pH is 1.5-2.
SECTION 10. STABILITY AND REACTIVITY
General: Stable.
Conditions to Avoid: May give off toxic fumes and possible oxides of constituents when exposed to Unusually high temperatures. Not established
Incompatible Materials: Avoid contact with bases and oxidizers.
Hazardous Decomposition/Combustion: Toxic fumes may form at elevated temperatures.
Hazardous Polymerization: Will not occur.
SECTION 11. TOXICOLOGICAL INFORMATION
Carcinogenicity:
National Toxicology Program (NTP): No
Occupational Safety & Health Administration (OSHA): No
U.N. International Agency for Research on Cancer (IARC): No
LD50: Not established.
LD50: Not established.
SECTION 12. ECOLOGICAL INFORMATION
Contains a substance that is harmful to aquatic organisms, and may cause long term adverse effects in the aquatic environment.
SECTION 13. DISPOSAL CONSIDERATIONS
Waste Disposal Method: Scrap indium metal may have some value. Contact a commercial reclaimer for recycling. Otherwise, dispose of in accordance with all Federal, State and Local environmental regulations. In Europe follow the Special Waste Regulations.
SECTION 14. TRANSPORT INFORMATION
Transport in accordance with applicable regulations and requirements.
Shipping Name: UN 3264,Corrosive Liquid, Acidic, Inorganic, N.O.S., 8, PGIII (sulfamic acid, indium sulfamate mixture)
North America Emergency Guidebook – Guide 154
Marine pollutant: No
SECTION 15. REGULATORY INFORMATION
The information in this Material Safety Data Sheet meets the requirements of the United States Occupational Safety and Health Act and regulations promulgated hereunder (29 CFR 1910.1200 ET. SEQ.).
This product has been classified in accordance with the hazard criteria of the Canadian Controlled Products Regulation (CPR).
Canadian WHMIS: Class E Corrosive Material
This product has been classified in accordance with the guidelines set by the Dept. of Industrial Health of the Republic of Singapore.
This product has been classified using the Chinese Occupational Exposure Limit of Hazardous Agents in the Workplace, GBZ2-2002.
This product has been classified in accordance with the Mexican regulations NOM-018-STPS-2000 and NOM-010-STPS-1999.
California Prop 65: Warning: This product contains a chemical known to the State of California to cause cancer and/or birth defects (or other reproductive harm). (There is a slight chance that trace levels of lead can be found).
SARA 313 listing - 40 CFR 372.65: None
All ingredients are listed on the EPA TSCA Inventory.
EC Classification, Packaging and Labeling Requirements: Symbol and Hazard Classification of Product Xn
Risk Phrases:
R20/21/22 Harmful by inhalation, in contact with skin and if swallowed
R36/37/38 Irritating to eyes, respiratory system and skin
R52/53 Harmful to aquatic organisms, may cause long term adverse effects in the aquatic environment.
Safety Phrases:
S20/21 When using do not eat, drink or smoke
S23 Do not breathe fumes/gas/vapor/spray
S24/25 Avoid contact with skin and eyes
S36/37/39 Wear suitable protective clothing, gloves and eye/face protection
S28 After contact with skin wash immediately with plenty of soap and water
SECTION 16. OTHER INFORMATION
Safety Data Sheet according to Regulation (EC) No. 1907/2006 (REACH). The above information is believed to be correct but does not purport to be all inclusive and shall be used only as a guide. The information in this document is based on the present state of our knowledge and is applicable to the product with regard to appropriate safety precautions. It does not represent any guarantee of the properties of the product. American Elements shall not be held liable for any damage resulting from handling or from contact with the above product. See reverse side of invoice or packing slip for additional terms and conditions of sale. COPYRIGHT 1997-2022 AMERICAN ELEMENTS. LICENSED GRANTED TO MAKE UNLIMITED PAPER COPIES FOR INTERNAL USE ONLY.
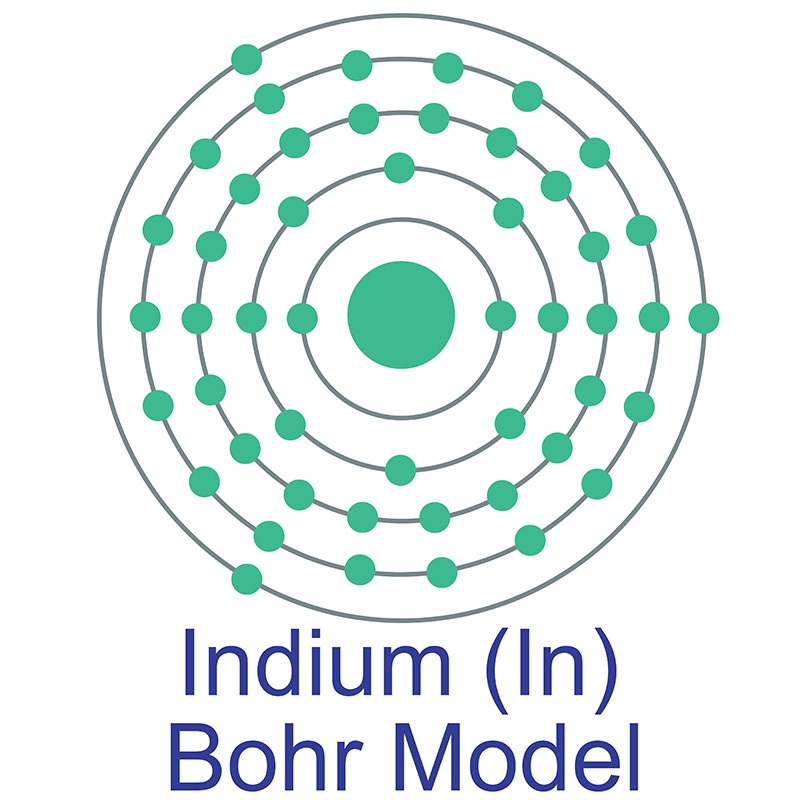 It is a relatively rare, extremely soft metal is a lustrous silvery gray and is both malleable and easily fusible. It has similar chemical properties to
It is a relatively rare, extremely soft metal is a lustrous silvery gray and is both malleable and easily fusible. It has similar chemical properties to  gallium such as a low melting point and the ability to wet glass. Fields such as optics and
gallium such as a low melting point and the ability to wet glass. Fields such as optics and 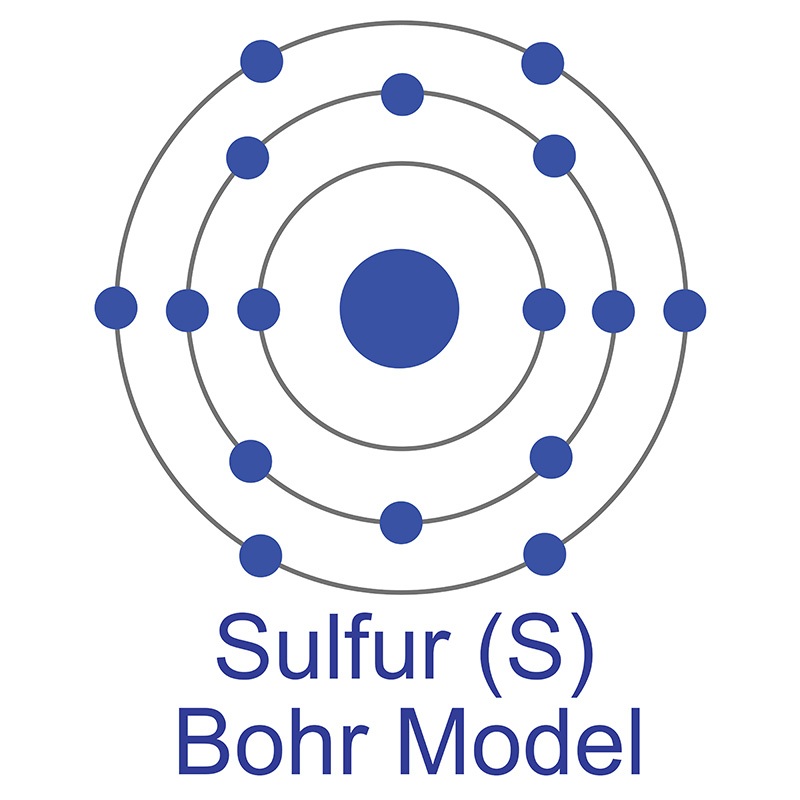 The number of electrons in each of Sulfur's shells is 2, 8, 6 and its electron configuration is [Ne] 3s2 3p4. In its elemental form, sulfur has a light yellow appearance. The sulfur atom has a covalent radius of 105 pm and a Van der Waals radius of 180 pm. In nature, sulfur can be found in hot springs, meteorites, volcanoes, and as galena, gypsum, and epsom salts. Sulfur has been known since ancient times but was not accepted as an element until 1777, when Antoine Lavoisier helped to convince the scientific community that it was an element and not a compound.
The number of electrons in each of Sulfur's shells is 2, 8, 6 and its electron configuration is [Ne] 3s2 3p4. In its elemental form, sulfur has a light yellow appearance. The sulfur atom has a covalent radius of 105 pm and a Van der Waals radius of 180 pm. In nature, sulfur can be found in hot springs, meteorites, volcanoes, and as galena, gypsum, and epsom salts. Sulfur has been known since ancient times but was not accepted as an element until 1777, when Antoine Lavoisier helped to convince the scientific community that it was an element and not a compound.