About Platinum
Pre-columbian natives of the Americas have produced items made from gold-platinum alloys for 2,000 years, but Spanish explorers first brought platinum to Europe in the 18th century in the form they called platina, the native form of the metal found in South America. The name was derived from plata, the Spanish word for silver metal, and was eventually used in the official name of the element. Antonio de Ulloa is credited with the discovery of platina in 1735, but was primarily an explorer and had little to do with investigation of the metal after submitting a report that included descriptions of the metal in 1746. Instead, a range of chemists across Europe experimented with the newly discovered metal.
18th century chemists recognized the potential value of the hard and corrosion-resistant metal but struggled to produce malleable platinum from the ore. In 1751, Swedish scientist Henrik Scheffer discovered that grains of the platinum ore could be fused into malleable platinum by heating them in the presence of arsenic, and subsequent refinement of this process enabled the production of the first European products made from the metal. Over the next fifty years, a variety of processes were developed to produce malleable platinum metal from ore, but all of them suffered from some inconsistencies in the final material produced and required laborious or costly steps, limiting the production of the metal. During this period, platinum was used primarily for the production of ornamental pieces and laboratory ware.
In 1802, English chemists Smithson Tennant and William Hyde Wollaston worked together to come up with a more efficient way to produce workable platinum. In the process, they discovered what had been hindering platinum purification all along: platinum ore actually contained trace amounts of several other elements previously unknown to science, and varying amounts of contamination with these elements remaining after extraction of the metal from the ore led to variations in the properties of the “platinum” produced. This new understanding facilitated the development of more reliable and efficient methods for processing platinum ore, which was followed by a boom in availability and use of the metal.
In 1817, Humphry Davy, a chemist who was interested in producing a lamp that could be safely used in coal mines, stumbled upon the phenomenon of heterogeneous catalytic oxidation: the coal gas he tested would burn without a flame and at a lower than usual temperature only when it came in contact with platinum or palladium metal wire. Having solved his problem, Davy then moved on to other pursuits, but other chemists were fascinated, and soon many reactions where platinum could serve as a catalyst were discovered. By the beginning of the 20th century, platinum catalysts were widely used in the industrial production of sulfuric and nitric acid.
Today, platinum catalysts are essential for catalytic converters, which reduce toxic emissions by automobiles, for petroleum processing, and for a wide range of organic synthesis applications. The 2007 Nobel Prize in Chemistry was awarded to Gerhard Ertl for the research on catalytic oxidation of carbon monoxide, the chemistry that underlies the function of catalytic converters. Additionally, two other Chemistry Nobel Prize winners investigated platinum catalysts in their research, though each was ultimately awarded the prize for processes that used other metals as catalysts. Paul Sabatier’s award in 1912 recognized him for his work on hydrogenation reactions for which he ultimately found nickel metal to be more effective than platinum. The first successful production of ammonia from gaseous nitrogen was performed in 1881 using a platinum catalyst, and Fritz Haber’s research into improving this process ultimately resulted in the Haber process for which he won the 1918 Nobel Prize. Haber’s final process as used in industry used iron-based catalysts rather than platinum group metals, but his research would not have been possible without prior work using platinum.
While researchers investigated the chemical properties of platinum in the 19th century, the metal was also growing in popularity for use in jewelry manufacturing. Platinum is in some ways a better metal for jewelry than either silver or gold, as it is harder than either and does not tarnish like silver. Once several prominent jewelers started using the metal in the late 1800’s, platinum rose rapidly in popularity, becoming particularly fashionable for the setting of colorless stones. This continued until 1940, when platinum use was restricted to industrial production of chemicals needed in the war effort. Platinum was replaced by white gold due to these restrictions, but has returned to popularity in recent years.
Platinum and platinum alloys are used in a wide range of settings where chemical inertness or wear resistance are important, including medical devices, laboratory instruments, electrical contacts, spark plugs, and turbine engines. A platinum-iridium alloy was also used to produce the international prototype kilogram and meter in the late 19th century; of the two only the kilogram remains in official use. Finally, organometallic platinum complexes have been investigated for use in cancer treatment.
Like other platinum group metals, platinum is most often obtained for commercial use as a byproduct from nickel and copper mining and processing, but can also be obtained from rare platinum-rich ores and alluvial deposits of native platinum.
Products
Platinum is used in laboratory and dentistry equipment, jewelry, electrical contacts and electrodes, and catalytic converters. It is highly corrosion resistant- the metal does not oxidize in air at any temperature. Organoplatinum compounds have been used as pharmaceutical treatments for certain cancers.  Platinum is available as metal and compounds with purities from 99% to 99.999% (ACS grade to ultra-high purity).
Platinum is available as metal and compounds with purities from 99% to 99.999% (ACS grade to ultra-high purity).  Elemental or metallic forms include platinum pellets, rod, wire and granules for evaporation source material purposes. Platinum nanoparticles and nanopowders are also available. Platinum oxide is available in powder and dense pellet form for such uses as optical coating and thin film applications. Oxides tend to be insoluble. Platinum fluoride is another insoluble form for uses in which oxygen is undesirable such as metallurgy, chemical and physical vapor deposition and in some optical coatings. Platinum is also available in soluble forms including chlorides, nitrates and acetates. These compounds can be manufactured as solutions at specified stoichiometries.
Elemental or metallic forms include platinum pellets, rod, wire and granules for evaporation source material purposes. Platinum nanoparticles and nanopowders are also available. Platinum oxide is available in powder and dense pellet form for such uses as optical coating and thin film applications. Oxides tend to be insoluble. Platinum fluoride is another insoluble form for uses in which oxygen is undesirable such as metallurgy, chemical and physical vapor deposition and in some optical coatings. Platinum is also available in soluble forms including chlorides, nitrates and acetates. These compounds can be manufactured as solutions at specified stoichiometries.
Platinum Properties
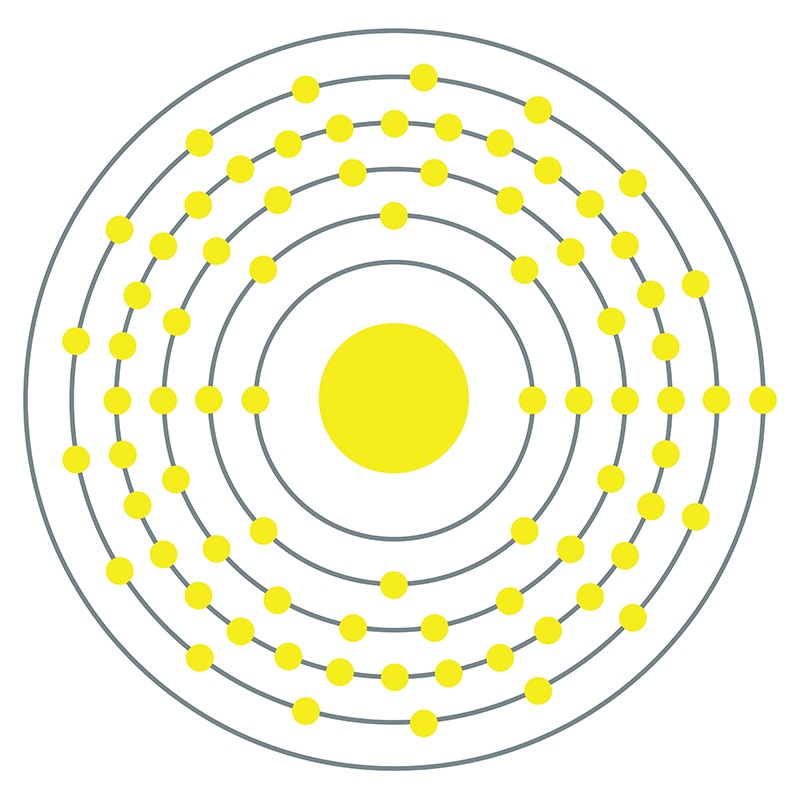
![]() Platinum is a Block D, Group 10, Period 6 element. The number of electrons in each of platinum's shells is 2, 8, 18, 32, 17, 1 and its electron configuration is [Xe] 4f14 5d9 6s1. The platinum atom has a radius of 137.3.pm and its Van der Waals radius is 175.pm.
Platinum is a Block D, Group 10, Period 6 element. The number of electrons in each of platinum's shells is 2, 8, 18, 32, 17, 1 and its electron configuration is [Xe] 4f14 5d9 6s1. The platinum atom has a radius of 137.3.pm and its Van der Waals radius is 175.pm. 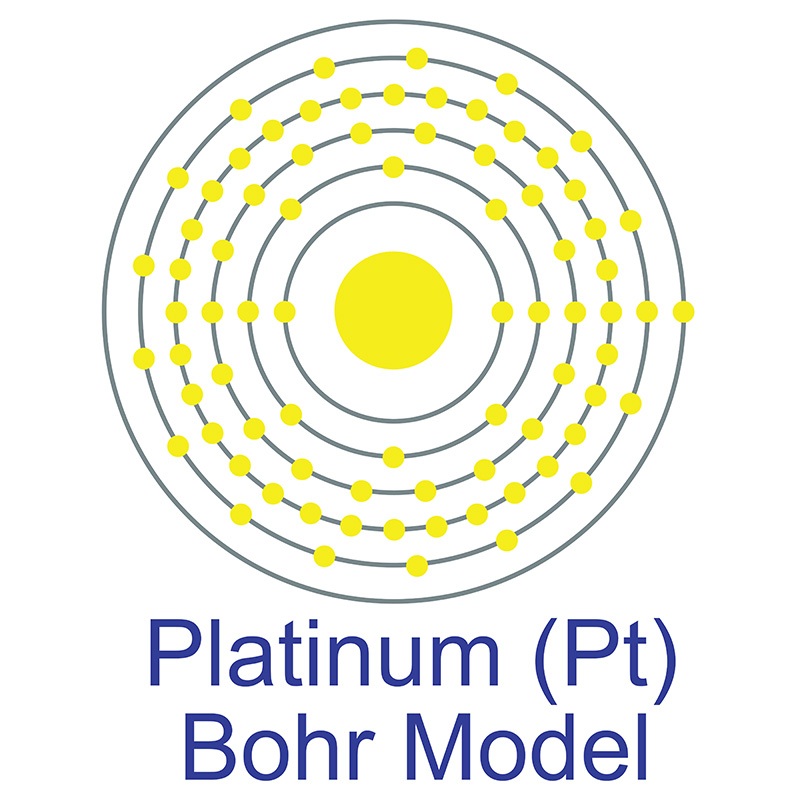
 In its elemental form, CAS 7440-06-4, platinum has a grayish white appearance. Platinum is a member of the platinum group of metals and the group 10 of the periodic table. It is generally non-reactive, even at high temperatures. It is one of the rarest elements in the earth's crust, occurring at a concentration of only 0.005 ppm. Platinum is found uncombined as native platinum and alloyed with iridium as platiniridium. Platinum was first discovered and isolated by Antonio de Ulloa in 1735. The origin of the name comes from the Spanish word platina meaning silver.
In its elemental form, CAS 7440-06-4, platinum has a grayish white appearance. Platinum is a member of the platinum group of metals and the group 10 of the periodic table. It is generally non-reactive, even at high temperatures. It is one of the rarest elements in the earth's crust, occurring at a concentration of only 0.005 ppm. Platinum is found uncombined as native platinum and alloyed with iridium as platiniridium. Platinum was first discovered and isolated by Antonio de Ulloa in 1735. The origin of the name comes from the Spanish word platina meaning silver.
Health, Safety & Transportation Information for Platinum
Platinum is not toxic in its elemental form; however, safety data for Platinum and its compounds can vary widely depending on the form. For potential hazard information, toxicity, and road, sea and air transportation limitations, such as DOT Hazard Class, DOT Number, EU Number, NFPA Health rating and RTECS Class, please see the specific material or compound referenced in the Products tab. The below information applies to elemental (metallic) Platinum.
| Safety Data | |
|---|---|
| Signal Word | N/A |
| Hazard Statements | N/A |
| Hazard Codes | N/A |
| Risk Codes | N/A |
| Safety Precautions | N/A |
| RTECS Number | TP2160000 |
| Transport Information | N/A |
| WGK Germany | nwg |
| Globally Harmonized System of Classification and Labelling (GHS) |
N/A |
Platinum Isotopes
Natural platinum (Pt) has five stable isotopes (192Pt, 194Pt, 195Pt, 196Pt, 198Pt) and one radioisotope with a very long half life (190Pt).
| Nuclide | Isotopic Mass | Half-Life | Mode of Decay | Nuclear Spin | Magnetic Moment | Binding Energy (MeV) | Natural Abundance (% by atom) |
|---|---|---|---|---|---|---|---|
| 166Pt | 165.99486(54)# | 300(100) µs | Unknown | 0+ | N/A | 1262.86 | - |
| 167Pt | 166.99298(44)# | 700(200) µs | Unknown | 7/2-# | N/A | 1270.94 | - |
| 168Pt | 167.98815(22) | 2.00(18) ms | a to 164Os; ß+ to 168Ir | 0+ | N/A | 1288.33 | - |
| 169Pt | 168.98672(22)# | 3.7(15) ms | a to 165Os; ß+ to 169Ir | 3/2-# | N/A | 1296.41 | - |
| 170Pt | 169.982495(20) | 14.0(2) ms | a to 166Os; ß+ to 170Ir | 0+ | N/A | 1304.49 | - |
| 171Pt | 170.98124(9) | 51(2) ms | a to 167Os; ß+ to 171Ir | 3/2-# | N/A | 1312.57 | - |
| 172Pt | 171.977347(14) | 98.4(24) ms | a to 168Os; ß+ to 172Ir | 0+ | N/A | 1329.97 | - |
| 173Pt | 172.97644(6) | 365(7) ms | a to 169Os; ß+ to 173Ir | 5/2-# | N/A | 1338.04 | - |
| 174Pt | 173.972819(13) | 0.889(17) s | a to 170Os; ß+ to 174Ir | 0+ | N/A | 1346.12 | - |
| 175Pt | 174.972421(20) | 2.53(6) s | a to 171Os; ß+ to 175Ir | 5/2-# | N/A | 1354.2 | - |
| 176Pt | 175.968945(15) | 6.33(15) s | ß+ to 176Ir; a to 172Os | 0+ | N/A | 1371.6 | - |
| 177Pt | 176.968469(16) | 10.6(4) s | ß+ to 177Ir; a to 173Os | 5/2- | N/A | 1379.68 | - |
| 178Pt | 177.965649(12) | 21.1(6) s | ß+ to 178Ir; a to 174Os | 0+ | N/A | 1387.75 | - |
| 179Pt | 178.965363(10) | 21.2(4) s | ß+ to 179Ir; a to 175Os | 1/2- | N/A | 1395.83 | - |
| 180Pt | 179.963031(12) | 56(2) s | ß+ to 180Ir; a to 176Os | 0+ | N/A | 1403.91 | - |
| 181Pt | 180.963097(16) | 52.0(22) s | ß+ to 181Ir; a to 177Os | 1/2- | N/A | 1411.99 | - |
| 182Pt | 181.961171(17) | 2.2(1) min | ß+ to 182Ir; a to 178Os | 0+ | N/A | 1420.07 | - |
| 183Pt | 182.961597(17) | 6.5(10) min | ß+ to 183Ir; a to 179Os | 1/2- | N/A | 1428.15 | - |
| 184Pt | 183.959922(19) | 17.3(2) min | ß+ to 184Ir; a to 180Os | 0+ | N/A | 1445.54 | - |
| 185Pt | 184.96062(4) | 70.9(24) min | ß+ to 185Ir; a to 181Os | (9/2+) | N/A | 1444.31 | - |
| 186Pt | 185.959351(23) | 2.08(5) h | ß+ to 186Ir; a to 182Os | 0+ | N/A | 1461.7 | - |
| 187Pt | 186.96059(3) | 2.35(3) h | ß+ to 187Ir | 3/2- | N/A | 1460.46 | - |
| 188Pt | 187.959395(6) | 10.2(3) d | EC to 188Ir; a to 184Os | 0+ | N/A | 1477.86 | - |
| 189Pt | 188.960834(12) | 10.87(12) h | ß+ to 189Ir | 3/2- | N/A | 1476.62 | - |
| 190Pt | 189.959932(6) | 6.5(3)E+11 y | a to 186Os | 0+ | N/A | 1494.01 | 0.014 |
| 191Pt | 190.961677(5) | 2.862(7) d | EC to 191Ir | 3/2- | 0.5 | 1492.78 | - |
| 192Pt | 191.9610380(27) | Observationally Stable | - | 0+ | N/A | 1500.86 | 0.782 |
| 193Pt | 192.9629874(18) | 50(6) y | EC to 193Ir | 1/2- | N/A | 1508.93 | - |
| 194Pt | 193.9626803(9) | Observationally Stable | - | 0+ | N/A | 1517.01 | 32.967 |
| 195Pt | 194.9647911(9) | Observationally Stable | - | 1/2- | 0.6095 | 1525.09 | 33.832 |
| 196Pt | 195.9649515(9) | Observationally Stable | - | 0+ | N/A | 1533.17 | 25.242 |
| 197Pt | 196.9673402(9) | 19.8915(19) h | ß- to 197Au | 1/2- | 0.51 | 1541.25 | - |
| 198Pt | 197.967893(3) | Observationally Stable | - | 0+ | N/A | 1549.33 | 7.163 |
| 199Pt | 198.970593(3) | 30.80(21) min | ß- to 199Au | 5/2- | N/A | 1548.09 | - |
| 200Pt | 199.971441(22) | 12.5(3) h | ß- to 200Au | 0+ | N/A | 1556.17 | - |
| 201Pt | 200.97451(5) | 2.5(1) min | ß- to 201Au | (5/2-) | N/A | 1564.25 | - |
| 202Pt | 201.97574(32)# | 44(15) h | ß- to 202Au | 0+ | N/A | 1572.33 | - |