About Rhenium
By the early twentieth century, what we know as the modern periodic table had largely taken shape. For the most part, what chemical discoveries remained would require the use of nuclear reactors rather than the traditional chemist’s patient examination and chemical analysis of unusual ores. There were, however, a few remaining holes in the table outside of the bottom rows that would later come to be filled with lab-produced radioactive elements; two of these missing elements were numbers 43 and 75.
Both of these elements had been predicted by Mendeleev, who had named them ekamanganese and dwimanganese respectively, but despite many attempts and declarations of discovery later deemed false, both elements remained elusive until 1924. In that year, the team of Walter Noddack and Ida Tacke, two German chemists who later married, with the assistance of Otto Berg, believed that they had found both of these missing elements in platinum ores, an assertion they based on spectral data. They named element 43 “masurian” for Noddack’s birthplace in the masurian marshes district, and element 75 “rhenium” from Tacke’s birthplace in Rhenany-Rhineland. Unfortunately, the team was only able to actually isolate substantial quantities of rhenium, and later theories convinced the scientific community that in fact, element 43 would be too unstable to be found by natural means. This lead to widespread disrespect for Tacke and Noddack as scientists, despite their verified discovery of one of the missing elements.
The early extraction and purification process for the rare element rhenium was complicated and expensive, which long delayed its industrial use. Even today, its applications are limited by its low availability, and is used primarily in functions where small quantities can provide substantial benefits. One of these functions is as an alloy additive: rhenium is a component of many heat-resistant superalloys. Nickel-based superalloys are frequently used in jet engines, where they are valued for high resistance to creep. Tungsten-rhenium and molybdenum-rhenium are used in thermocouples most often deployed for sensing extremely high temperatures. Rhenium-containing alloys may also find use in crucibles, self-cleaning electrical contacts, electromagnets, ionization gauges, and mass spectrographs. The other major use of rhenium is as a catalyst; it serves this function in the processing of petroleum, alongside platinum in catalytic converters, and in a number of niche or still developing applications. Additionally, Re-188 and Re-186 are used in cancer radiotherapy.
Rhenium is most often found in small quantities in molybdenum deposits, and is obtained through processing of molybdenum concentrates. Additionally, platinum-rhenium catalysts and rhenium alloy scrap are frequently recycled.
Products
High-temperature rhenium super-alloys are used to make jet engine parts. Platinum-rhenium catalysts are used in lead-free, high-octane, gasoline. Rhenium is also used as a filament for mass spectrographs and ion gauges. Because rhenium has good wear resistance and withstands arc corrosion, it is used as an electrical  contact material. Thermocouples made of Re-W are used for measuring temperatures up to 2200C, and rhenium wire is used in photographic flash lamps. Rhenium is available as metal and compounds with purities from 99% to 99.999% (ACS grade to ultra-high purity).
contact material. Thermocouples made of Re-W are used for measuring temperatures up to 2200C, and rhenium wire is used in photographic flash lamps. Rhenium is available as metal and compounds with purities from 99% to 99.999% (ACS grade to ultra-high purity).  Elemental or metallic forms include pellets, rod, wire and granules for evaporation source material purposes. Rhenium nanoparticles and nanopowders provide ultra-high surface area. Rhenium oxides are available in powder and dense pellet form for such uses as optical coating and thin film applications. Oxides tend to be insoluble. Rhenium fluorides are another insoluble form for uses in which oxygen is undesirable such as metallurgy, chemical and physical vapor deposition and in some optical coatings. Rhenium is also available in soluble forms including as its chlorides. These compounds can be manufactured as solutions at specified stoichiometries.
Elemental or metallic forms include pellets, rod, wire and granules for evaporation source material purposes. Rhenium nanoparticles and nanopowders provide ultra-high surface area. Rhenium oxides are available in powder and dense pellet form for such uses as optical coating and thin film applications. Oxides tend to be insoluble. Rhenium fluorides are another insoluble form for uses in which oxygen is undesirable such as metallurgy, chemical and physical vapor deposition and in some optical coatings. Rhenium is also available in soluble forms including as its chlorides. These compounds can be manufactured as solutions at specified stoichiometries.
Rhenium Properties
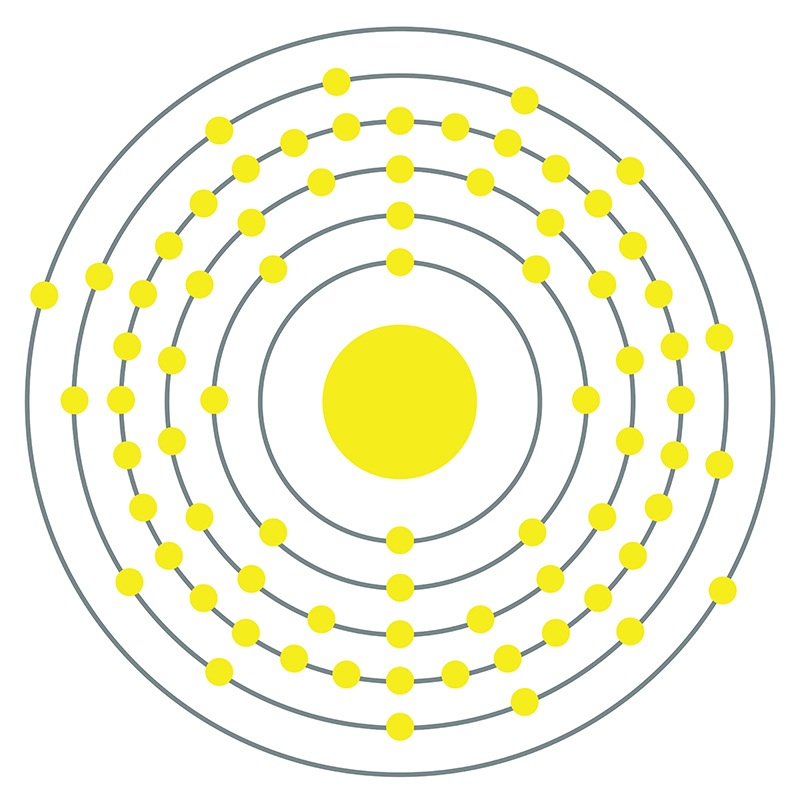
 Rhenium is a Block D, Group 7, Period 6 element. The number of electrons in each of rhenium's shells is 2, 8, 18, 32, 13, 2 and its electron configuration is [Xe] 4f14 5d5 6s2.
Rhenium is a Block D, Group 7, Period 6 element. The number of electrons in each of rhenium's shells is 2, 8, 18, 32, 13, 2 and its electron configuration is [Xe] 4f14 5d5 6s2. 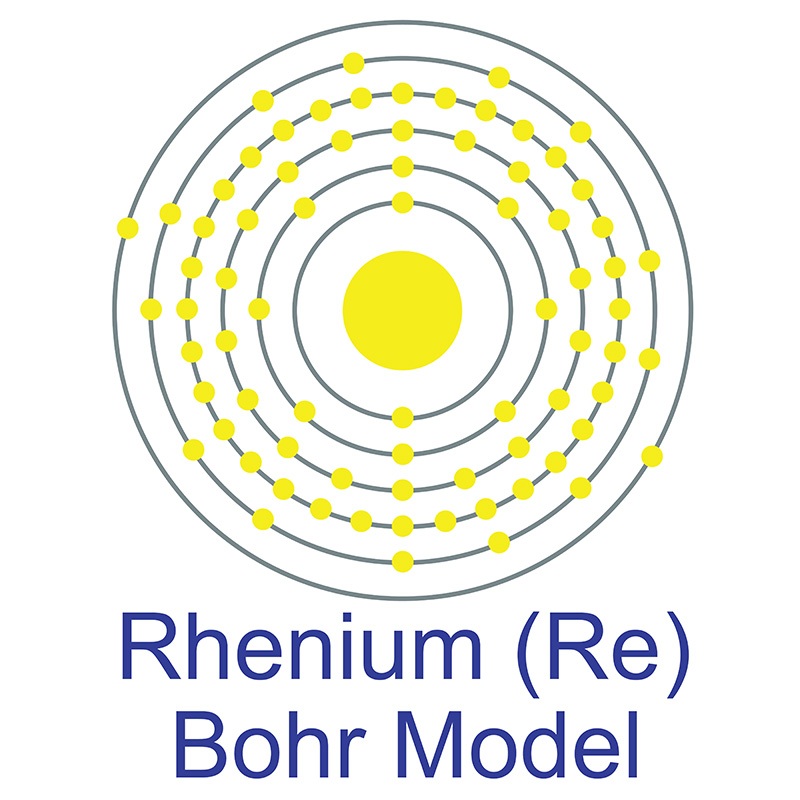
Health, Safety & Transportation Information for Rhenium
Very little is known about the toxicity of rhenium and its compounds. Safety data for Rhenium and its compounds can vary widely depending on the form. For potential hazard information, toxicity, and road, sea and air transportation limitations, such as DOT Hazard Class, DOT Number, EU Number, NFPA Health rating and RTECS Class, please see the specific material or compound referenced in the Products tab. The below information applies to elemental (metallic) Rhenium.
| Safety Data | |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H228 |
| Hazard Codes | F |
| Risk Codes | 11 |
| Safety Precautions | 16 |
| RTECS Number | VI0780000 |
| Transport Information | N/A |
| WGK Germany | nwg |
| Globally Harmonized System of Classification and Labelling (GHS) |
|
Rhenium Isotopes
Naturally occurring rhenium (Re) has two isotopes: 185Re (37.4%) and 187Re (62.6%) .
| Nuclide | Isotopic Mass | Half-Life | Mode of Decay | Nuclear Spin | Magnetic Moment | Binding Energy (MeV) | Natural Abundance (% by atom) |
|---|---|---|---|---|---|---|---|
| 160Re | 159.98212(43)# | 860(120) µs [0.82(+15-9) ms] | p to 159W; a to 156Ta | (2-) | N/A | 1227.07 | - |
| 161Re | 160.97759(22) | 0.37(4) ms | p to 160W | 1/2+ | N/A | 1244.47 | - |
| 162Re | 161.97600(22)# | 107(13) ms | a to 158Ta; ß+ to 162W | (2-) | N/A | 1252.54 | - |
| 163Re | 162.972081(21) | 390(70) ms | ß+ to 163W; a to 159Ta | (1/2+) | N/A | 1260.62 | - |
| 164Re | 163.97032(17)# | 0.53(23) s | a to 160Ta; ß+ to 164W | high | N/A | 1268.7 | - |
| 165Re | 164.967089(30) | 1# s | ß+ to 165W; a to 161Ta | 1/2+# | N/A | 1286.1 | - |
| 166Re | 165.96581(9)# | 2# s | ß+ to 166W; a to 162Ta | 2-# | N/A | 1294.18 | - |
| 167Re | 166.96260(6)# | 3.4(4) s | a to 163Ta; ß+ to 167W | 9/2-# | N/A | 1302.25 | - |
| 168Re | 167.96157(3) | 4.4(1) s | ß+ to 168W; a to 164Ta | (5+,6+,7+) | N/A | 1310.33 | - |
| 169Re | 168.95879(3) | 8.1(5) s | ß+ to 169W; a to 165Ta | 9/2-# | N/A | 1327.73 | - |
| 170Re | 169.958220(28) | 9.2(2) s | ß+ to 170W; a to 166Ta | (5+) | N/A | 1335.81 | - |
| 171Re | 170.95572(3) | 15.2(4) s | ß+ to 171W | (9/2-) | N/A | 1343.89 | - |
| 172Re | 171.95542(6) | 15(3) s | ß+ to 172W | -5 | N/A | 1351.96 | - |
| 173Re | 172.95324(3) | 1.98(26) min | ß+ to 173W | (5/2-) | N/A | 1360.04 | - |
| 174Re | 173.95312(3) | 2.40(4) min | ß+ to 174W | N/A | N/A | 1368.12 | - |
| 175Re | 174.95138(3) | 5.89(5) min | ß+ to 175W | (5/2-) | N/A | 1376.2 | - |
| 176Re | 175.95162(3) | 5.3(3) min | ß+ to 176W | 3+ | N/A | 1384.28 | - |
| 177Re | 176.95033(3) | 14(1) min | ß+ to 177W | 5/2- | N/A | 1392.36 | - |
| 178Re | 177.95099(3) | 13.2(2) min | ß+ to 178W | (3+) | N/A | 1400.44 | - |
| 179Re | 178.949988(26) | 19.5(1) min | ß+ to 179W | (5/2)+ | N/A | 1417.83 | - |
| 180Re | 179.950789(23) | 2.44(6) min | ß+ to 180W | (1)- | N/A | 1416.59 | - |
| 181Re | 180.950068(14) | 19.9(7) h | ß+ to 181W | 5/2+ | N/A | 1424.67 | - |
| 182Re | 181.95121(11) | 64.0(5) h | EC to 182W | 7+ | 2.8 | 1432.75 | - |
| 183Re | 182.950820(9) | 70.0(14) d | EC to 183W | 5/2+ | 3.17 | 1440.83 | - |
| 184Re | 183.952521(5) | 38.0(5) d | EC to 184W | 3(-) | 2.53 | 1448.91 | - |
| 185Re | 184.9529550(13) | Observationally Stable | - | 5/2+ | 3.1871 | 1456.99 | 37.4 |
| 186Re | 185.9549861(13) | 3.7186(5) d | EC to 186W; ß- to 186Os | 1- | 1.739 | 1465.07 | - |
| 187Re | 186.9557531(15) | 41.2(2)E+9 y | ß- to 187Os; a to 183Ta | 5/2+ | 3.2197 | 1473.15 | 62.6 |
| 188Re | 187.9581144(15) | 17.0040(22) h | ß- to 188Os | 1- | 1.788 | 1481.22 | - |
| 189Re | 188.959229(9) | 24.3(4) h | ß- to 189Os | 5/2+ | N/A | 1489.3 | - |
| 190Re | 189.96182(16) | 3.1(3) min | ß- to 190Os | (2)- | N/A | 1488.06 | - |
| 191Re | 190.963125(11) | 9.8(5) min | ß- to 191Os | (3/2+,1/2+) | N/A | 1496.14 | - |
| 192Re | 191.96596(21)# | 16(1) s | ß- to 192Os | N/A | N/A | 1504.22 | - |
| 193Re | 192.96747(21)# | 30# s [>300 ns] | Unknown | 5/2+# | N/A | 1512.3 | - |
| 194Re | 193.97042(32)# | 2# s [>300 ns] | Unknown | N/A | N/A | 1511.06 | - |