SECTION 1. IDENTIFICATION
Product Name: Silver Iodide
Product Number: All applicable American Elements product codes, e.g. AG1-I-02
, AG1-I-03
, AG1-I-04
, AG1-I-05
CAS #: 7783-96-2
Relevant identified uses of the substance: Scientific research and development
Supplier details:
American Elements
10884 Weyburn Ave.
Los Angeles, CA 90024
Tel: +1 310-208-0551
Fax: +1 310-208-0351
Emergency telephone number:
Domestic, North America: +1 800-424-9300
International: +1 703-527-3887
SECTION 2. HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Appearance: light yellow solid.
Caution! May cause eye and skin irritation. May cause respiratory and digestive tract irritation. Light sensitive. May cause reproductive and fetal effects. The toxicological properties of this material have not been fully investigated.
Target Organs: Thyroid.
Potential Health Effects
Eye: May cause eye irritation.
Skin: May cause skin irritation. Can cause eczema and rash.
Ingestion: May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated. Chronic ingestion of iodides during pregnancy has resulted in fetal death, severe goiter, and cretinoid appearance of the newborn.
Inhalation: May cause respiratory tract irritation. May cause effects similar to those described for ingestion. The toxicological properties of this substance have not been fully investigated.
Chronic: Chronic inhalation or ingestion of silver salts may cause argyria characterized by a permanent blue-gray discoloration of the eyes, skin, mucous membranes, and internal organs. This malady results from the accumulation of silver in the body. Chronic ingestion of iodides during pregnancy has resulted in fetal death, severe goiter, and cretinoid appearance of the newborn. Prolonged exposure to iodides may produce iodism in sensitive individuals. Symptoms could include skin rash, running nose and headache.
SECTION 3. COMPOSITION/INFORMATION ON INGREDIENTS
CAS# 7783-96-2
Chemical Name Silver Iodide
EINECS/ELINCS 232-038-0
SECTION 4. FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin: Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion: Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation: Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician: Treat symptomatically and supportively.
SECTION 5. FIREFIGHTING MEASURES
General Information: As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Non-combustible, substance itself does not burn but may decompose upon heating to produce irritating, corrosive and/or toxic fumes.
Extinguishing Media: Substance is noncombustible; use agent most appropriate to extinguish surrounding fire. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Flash Point: N/A
Autoignition Temperature: N/A
Explosion Limits, Lower:Not available.
Upper: Not available.
NFPA Rating: (estimated) Health: 1; Flammability: 0; Instability: 0
SECTION 6. ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks: Vacuum or sweep up material and place into a suitable disposal container. Avoid runoff into storm sewers and ditches which lead to waterways. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions. Provide ventilation.
SECTION 7. HANDLING AND STORAGE
Handling: Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation. Store protected from light.
Storage: Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances. Store protected from light.
SECTION 8. EXPOSURE CONTROLS/PERSONAL PROTECTION
Engineering Controls: Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits
Chemical Name ACGIH NIOSH OSHA - Final PELs
Silver Iodide none listed none listed none listed
OSHA Vacated PELs: Silver Iodide: No OSHA Vacated PELs are listed for this chemical.
Personal Protective Equipment
Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin: Wear appropriate protective gloves to prevent skin exposure.
Clothing: Wear appropriate protective clothing to prevent skin exposure.
Respirators: A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
SECTION 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Appearance: light yellow
Odor: none reported
pH: Not available.
Vapor Pressure: Negligible.
Vapor Density: Not available.
Evaporation Rate:negligible
Viscosity: Not available.
Boiling Point: N/A
Freezing/Melting Point:552.2 deg C
Decomposition Temperature:Not available.
Solubility: Slightly soluble in water.
Specific Gravity/Density:5.68 @ 30°C
Molecular Formula:AgI
Molecular Weight:234.7727
SECTION 10. STABILITY AND REACTIVITY
Chemical Stability: Stable under normal temperatures and pressures.
Conditions to Avoid: High temperatures, incompatible materials, light, dust generation.
Incompatibilities with Other Materials: Strong oxidizing agents.
Hazardous Decomposition Products: Irritating and toxic fumes and gases, hydrogen iodide.
Hazardous Polymerization: Has not been reported
SECTION 11. TOXICOLOGICAL INFORMATION
RTECS#: VW4450000
LD50/LC50:
Not available.
Carcinogenicity:
CAS# 7783-96-2: Not listed by ACGIH, IARC, NTP, or CA Prop 65.
Epidemiology: No information found
Teratogenicity: No information found
Reproductive Effects: No information found
Mutagenicity: No information found
Neurotoxicity: No information found
Other Studies:
Chemical waste generators must determine whether a discarded chemical is classified as a hazardous waste. US EPA guidelines for the classification determination are listed in 40 CFR Parts 261.3. Additionally, waste generators must consult state and local hazardous waste regulations to ensure complete and accurate classification.
RCRA P-Series: None listed.
RCRA U-Series: None listed.
SECTION 12. ECOLOGICAL INFORMATION
No data available.
SECTION 13. DISPOSAL CONSIDERATIONS
Chemical waste generators must determine whether a discarded chemical is classified as a hazardous waste. US EPA guidelines for the classification determination are listed in 40 CFR Parts 261.3. Additionally, waste generators must consult state and local hazardous waste regulations to ensure complete and accurate classification.
RCRA P-Series: None listed.
RCRA U-Series: None listed.
SECTION 14. TRANSPORT INFORMATION
DOT (US)
Not dangerous goods
IMDG
UN number: 3077 Class: 9 Packing group: III EMS-No: F-A, S-F
Proper shipping name: ENVIRONMENTALLY HAZARDOUS SUBSTANCE, SOLID, N.O.S. (Silver iodide)
Marine pollutant:yes
IATA
UN number: 3077 Class: 9 Packing group: III
Proper shipping name: Environmentally hazardous substance, solid, n.o.s. (Silver iodide)
Further information
EHS-Mark required (ADR 2.2.9.1.10, IMDG code 2.10.3) for single packagings and combination packagings containing
inner packagings with Dangerous Goods > 5L for liquids or > 5kg for solids.
SECTION 15. REGULATORY INFORMATION
US FEDERAL
TSCA
CAS# 7783-96-2 is listed on the TSCA inventory.
Health & Safety Reporting List
None of the chemicals are on the Health & Safety Reporting List.
Chemical Test Rules
None of the chemicals in this product are under a Chemical Test Rule.
Section 12b
None of the chemicals are listed under TSCA Section 12b.
TSCA Significant New Use Rule
None of the chemicals in this material have a SNUR under TSCA.
CERCLA Hazardous Substances and corresponding RQs
None of the chemicals in this material have an RQ.
SARA Section 302 Extremely Hazardous Substances
None of the chemicals in this product have a TPQ.
SARA Codes
CAS # 7783-96-2: delayed.
Section 313
This material contains Silver Iodide (listed as Silver compounds), 99%, (CAS# 7783-96-2) which is subject to the reporting requirements of Section 313 of SARA Title III and 40 CFR Part 373.
Clean Air Act:
This material does not contain any hazardous air pollutants.
This material does not contain any Class 1 Ozone depletors.
This material does not contain any Class 2 Ozone depletors.
Clean Water Act:
None of the chemicals in this product are listed as Hazardous Substances under the CWA.
None of the chemicals in this product are listed as Priority Pollutants under the CWA. CAS# 7783-96-2 is listed as a Toxic Pollutant under the Clean Water Act.
OSHA:
None of the chemicals in this product are considered highly hazardous by OSHA.
STATE
CAS# 7783-96-2 can be found on the following state right to know lists: California, (listed as Silver compounds), New Jersey, (listed as Silver compounds), Pennsylvania, (listed as Silver compounds).
California Prop 65
California No Significant Risk Level: None of the chemicals in this product are listed.
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols:
Not available.
Risk Phrases:
Safety Phrases:
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek medical advice
immediately (show the label where possible).
S 28A After contact with skin, wash immediately with plenty of water
.
WGK (Water Danger/Protection)
CAS# 7783-96-2: 1
Canada - DSL/NDSL
CAS# 7783-96-2 is listed on Canada's DSL List.
Canada - WHMIS
This product has a WHMIS classification of D2A.
This product has been classified in accordance with the hazard criteria of the Controlled Products Regulations and the MSDS contains all of the information required by those regulations.
Canadian Ingredient Disclosure List
CAS# 7783-96-2 is not listed on the Canadian Ingredient Disclosure List
SECTION 16. OTHER INFORMATION
Safety Data Sheet according to Regulation (EC) No. 1907/2006 (REACH). The above information is believed to be correct but does not purport to be all inclusive and shall be used only as a guide. The information in this document is based on the present state of our knowledge and is applicable to the product with regard to appropriate safety precautions. It does not represent any guarantee of the properties of the product. American Elements shall not be held liable for any damage resulting from handling or from contact with the above product. See reverse side of invoice or packing slip for additional terms and conditions of sale. COPYRIGHT 1997-2022 AMERICAN ELEMENTS. LICENSED GRANTED TO MAKE UNLIMITED PAPER COPIES FOR INTERNAL USE ONLY.

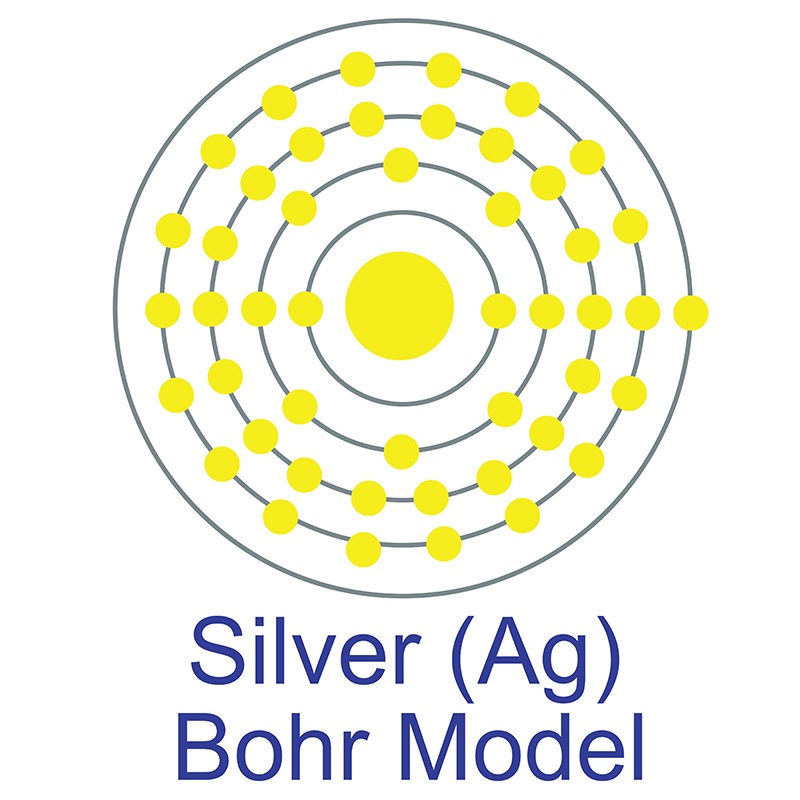 The number of electrons in each of Silver's shells is 2, 8, 18, 18, 1 and its electron configuration is [Kr]4d10 5s1. The silver atom has a radius of 144 pm and a Van der Waals radius of 203 pm. Silver was first discovered by Early Man prior to 5000 BC. In its elemental form, silver has a brilliant white metallic luster.
The number of electrons in each of Silver's shells is 2, 8, 18, 18, 1 and its electron configuration is [Kr]4d10 5s1. The silver atom has a radius of 144 pm and a Van der Waals radius of 203 pm. Silver was first discovered by Early Man prior to 5000 BC. In its elemental form, silver has a brilliant white metallic luster.  It is a little harder than
It is a little harder than 
