About Tantalum
Tantalum is extremely chemically similar to niobium; the two exist neither as free elements on earth nor independent from each other, present in minerals like tantalite, columbite, samarskite, microlite, wodginite, euxenite, fergusonite, and polycrase. The minerals called tantalite and columbite (alternately, niobate) actually have the same chemical structure, the two names reflecting a difference in relative proportion of tantalum to niobium. The history of the two elements is similarly entwined. In 1801, British chemist and mineralogist Charles Hatchett became intrigued by a mineral sample displayed in the collection that had been sent decades earlier from the American colonies by Connecticut governor John Winthrop. His analysis of the sample yielded a substance he believed contained a heretofore undiscovered element that he named “columbium” for Columbia, the symbolic female embodiment of the United States, and the mineral itself came to be called columbite. Less than a year later in Uppsala, Sweden, chemist Anders Gustaf Ekeberg suspected he had discovered a new element by similarly extracting an oxide form from tantalite. This oxide proved so highly resistant to attack by even acid that he named the element “tantalum” after the Greek mythological figure of Tantalus, who remained unable to quench his thirst from the water in which he stood. Later that year, however, British chemist William Hyde Wollaston declared that columbium and tantalum were in fact the same element (despite the difference in density between the two oxide forms), and that tantalum should be its official name.
Much confusion ensued in the following years within the scientific community. In 1845, German chemist Heinrich Rose posited that tantalite contained two additional elements besides tantalum, naming them “pelopium” and “niobium” after Pelops and Niobe, the mythological daughters of Tantalus; in 1847, R. Hermann announced that he had discovered a new element, “ilmenium,” from the mineral samarskite. Pelopium and ilmenium were subsequently shown to be mixes of tantalum and niobium. The difference between tantalum and niobium was not empirically proven until the 1860’s by Jean Charles Galissard de Marignac, and pure tantalum was not isolated until 1903 by Werner von Bolton. For years, the standard method for separating the two elements was achieved via de Marignac’s method of fractional crystallization; later methods developed include liquid-liquid extraction from aqueous solutions of fluoride compounds and a modified version of the Hall-Héroult electrolysis technique based on powdered oxides.
Elemental tantalum is a lustrous blue-gray transition metal that is a member of the heat-resistant refractory metal group. Naturally occurring tantalum has two isotopes; metastable 180m-Ta is both the only naturally-occurring nuclear isomer and is also calculated to be the rarest primordial isotope in the universe. Tantalum metal is hard and dense, yet also ductile and highly malleable; its resistance to corrosion is similar to that of glass and the highest of any metal in common use. An extremely thin and stable surface film of tantalum pentoxide provides the metal with its surface passivation: it is essentially chemically inert at temperatures below 150 C and resists attack by all acids except hydrofluoric acid, fluoride-containing acidic solutions, and oleum (sulfur trioxide in sulfuric acid solution). This stability and non-reactivity with body tissue or fluids makes it useful for chemical and pharmaceutical components, laboratory instruments, and medical implants, especially as a more cost-effective substitute for platinum and other precious metals. Tantalum has one of the highest melting points of all elements, surpassed only by tungsten, rhenium, osmium, and carbon, yet it is also highly conductive to heat and electricity. Coupled with its extreme resistance to corrosion, these properties make tungsten a critical industrial metal for applications in high-temperature oxidizing environments like linings of nuclear reactors, aircraft components, rocket engines, vacuum furnace and heat exchanger installations, and chemical processing equipment. The metal was also used in early lamp filaments and radio transmitter electron tubes for this reason. Tantalum alloys and superalloys are extremely dense, strong, and durable, with high melting points and excellent machinability that are utilized by the aerospace, nuclear, defense, and hot metal-working industries. Other significant industrial materials are tantalum nitrides and tantalum carbides: hard, durable, and highly heat-resistant ceramics materials used in cutting tools, jet engine and turbine blades, and drill bits. A tantalum carbide-graphite composite material developed by scientists at the Los Alamos National Lab has been labeled one of the hardest materials ever synthesized.
The most prevalent commercial use of metallic tantalum is in the compact, high-performance capacitors of electronics such as mobile devices, digital cameras, laptop computers, pacemakers, GPS systems, and anti-lock braking systems. Because dielectric coatings of tantalum pentoxide are thinner than their aluminum corollary, tantalum-based capacitors are lighter and more efficient, in addition to being highly reliable with low leakage and excellent capacity. Materials like tantalum nitride have also been used in thin-chip resistors and as copper diffusion barriers in micro and nanoelectronic devices. Other applications for tantalum have emerged in advanced technology. Lithium tantalate (LiTaO3) is a ferroelectric perovskite crystal used in non-linear optics, and the high refractive index of tantalum oxide is utilized in optical coatings and metallic glass. In addition, the metal’s excellent resistance to corrosion and heat make it an attractive cost-effective alternative to platinum or gold in fuel cell collector plates and in the extreme environments required for production of alternative energy sources like biofuels.
Products
Tantalum has a number of interesting properties that make it particularly useful in electronic applications. It has the third highest melting point, surpassed only by rhenium and tungsten, yet it is highly conductive to heat and electricity. This has made it the material of choice for the electronic capacitors used in most telecommunications and hand held electronics equipment, such as cell phones, laptop computers and pagers. Tantalum compounds, such as tantalum oxide and tantalum chloride, are the basis for dielectric coatings.  Tantalum is added to glass for its high refractive index. It also has various applications in nuclear energy. Tantalum is available as metal and compounds with purities from 99% to 99.999% (ACS grade to ultra-high purity). Elemental or metallic forms include tantalum pellets, rod, wire and granules for evaporation source material purposes.
Tantalum is added to glass for its high refractive index. It also has various applications in nuclear energy. Tantalum is available as metal and compounds with purities from 99% to 99.999% (ACS grade to ultra-high purity). Elemental or metallic forms include tantalum pellets, rod, wire and granules for evaporation source material purposes.  Tantalum nanoparticles and nanopowders provide ultra-high surface area which nanotechnology research and recent experiments demonstrate function to create new and unique properties and benefits. Oxides are available in powder and dense pellet form for such uses as optical coating and thin film applications. Oxides tend to be insoluble. Fluorides are another insoluble form for uses in which oxygen is undesirable such as metallurgy, chemical and physical vapor deposition and in some optical coatings. Tantalum is also available in soluble forms including chloride, nitrate, and acetate. These compounds can be manufactured as solutions at specified stoichiometries.
Tantalum nanoparticles and nanopowders provide ultra-high surface area which nanotechnology research and recent experiments demonstrate function to create new and unique properties and benefits. Oxides are available in powder and dense pellet form for such uses as optical coating and thin film applications. Oxides tend to be insoluble. Fluorides are another insoluble form for uses in which oxygen is undesirable such as metallurgy, chemical and physical vapor deposition and in some optical coatings. Tantalum is also available in soluble forms including chloride, nitrate, and acetate. These compounds can be manufactured as solutions at specified stoichiometries.
American Elements assists our Tantalum customers with fulfilling the due diligence reporting requirements of the Conflict Mineral Provision (Section 1502) of the Dodd-Frank Act.
Tantalum Properties
 Tantalum is a Block D, Group 5, Period 6 element. The number of electrons in each of tantalum's shells is 2, 8, 18, 32, 11, 2 and its electron configuration is [Xe] 4f14 5d3 6s2.
Tantalum is a Block D, Group 5, Period 6 element. The number of electrons in each of tantalum's shells is 2, 8, 18, 32, 11, 2 and its electron configuration is [Xe] 4f14 5d3 6s2. 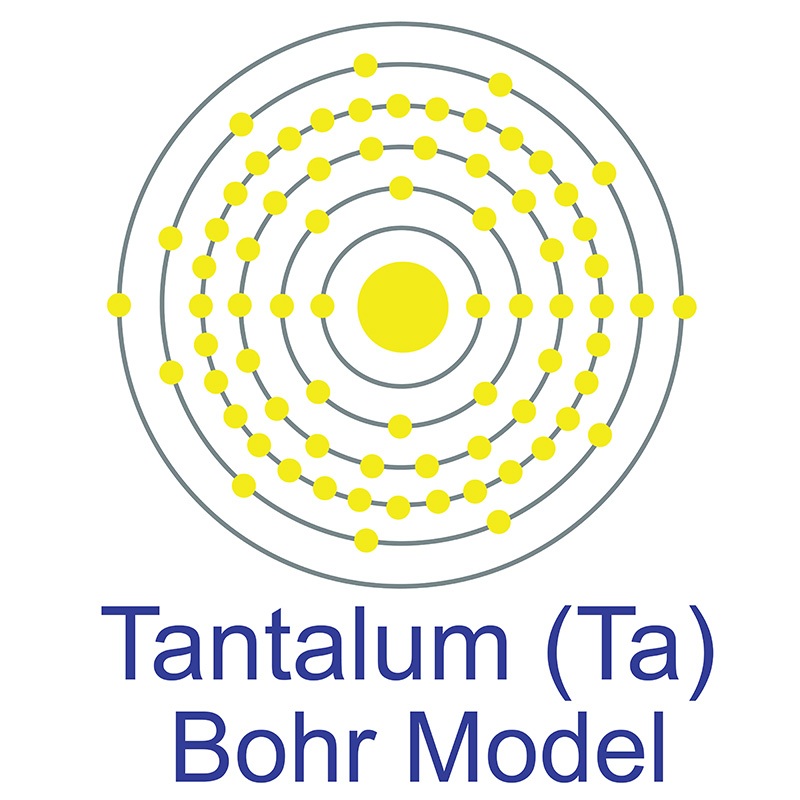 The tantalum atom has a radius of 143.pm and its Van der Waals radius is 200.pm. In its elemental form, CAS 7440-25-7, tantalum has a gray blue appearance.
The tantalum atom has a radius of 143.pm and its Van der Waals radius is 200.pm. In its elemental form, CAS 7440-25-7, tantalum has a gray blue appearance.  Tantalum is found in the minerals tantalite, microlite, wodginite, euxenite, and polycrase. Tantalum was first discovered by Anders G. Ekeberg in 1802 in Uppsala, Sweden. Due to the close relation of tantalum to niobium in the periodic table, Tantalum's name originates from the Greek word Tantalos, meaning Father of Niobe in Greek mythology.
Tantalum is found in the minerals tantalite, microlite, wodginite, euxenite, and polycrase. Tantalum was first discovered by Anders G. Ekeberg in 1802 in Uppsala, Sweden. Due to the close relation of tantalum to niobium in the periodic table, Tantalum's name originates from the Greek word Tantalos, meaning Father of Niobe in Greek mythology.
Health, Safety & Transportation Information for Tantalum
Tantalum is not toxic in its elemental form; however, safety data for Tantalum and its compounds can vary widely depending on the form. For potential hazard information, toxicity, and road, sea and air transportation limitations, such as DOT Hazard Class, DOT Number, EU Number, NFPA Health rating and RTECS Class, please see the specific material or compound referenced in the Products tab. The below information applies to elemental (metallic) Tantalum.
| Safety Data | |
|---|---|
| Signal Word | N/A |
| Hazard Statements | N/A |
| Hazard Codes | N/A |
| Risk Codes | N/A |
| Safety Precautions | N/A |
| RTECS Number | N/A |
| Transport Information | N/A |
| WGK Germany | nwg |
| Globally Harmonized System of Classification and Labelling (GHS) |
N/A |
Tantalum Isotopes
Naturally occuring tantalum (Ta) has two stable isotopes: 180Ta (0.012%) and 181Ta (99.988%).
| Nuclide | Isotopic Mass | Half-Life | Mode of Decay | Nuclear Spin | Magnetic Moment | Binding Energy (MeV) | Natural Abundance (% by atom) |
|---|---|---|---|---|---|---|---|
| 155Ta | 154.97459(54)# | 13(4) µs [12(+4-3) µs] | Unknown | (11/2-) | N/A | 1198.24 | - |
| 156Ta | 155.97230(43)# | 144(24) ms | ß+ to 156Hf; p to 155Lu | (2-) | N/A | 1206.32 | - |
| 157Ta | 156.96819(22) | 10.1(4) ms | a to 153Lu; ß+ to 157Hf | 1/2+ | N/A | 1223.71 | - |
| 158Ta | 157.96670(22)# | 49(8) ms | a to 154Lu; ß+ to 158Hf | (2-) | N/A | 1231.79 | - |
| 159Ta | 158.963018(22) | 1.04(9) s | ß+ to 159Hf; a to 155Lu | (1/2+) | N/A | 1239.87 | - |
| 160Ta | 159.96149(10) | 1.70(20) s | a to 156Lu; ß+ to 160Hf | (2#)- | N/A | 1247.95 | - |
| 161Ta | 160.95842(6)# | 3# s | ß+ to 161Hf; a to 157Lu | 1/2+# | N/A | 1265.34 | - |
| 162Ta | 161.95729(6) | 3.57(12) s | ß+ to 162Hf; a to 158Lu | 3+# | N/A | 1273.42 | - |
| 163Ta | 162.95433(4) | 10.6(18) s | ß+ to 163Hf; a to 159Lu | 1/2+# | N/A | 1281.5 | - |
| 164Ta | 163.95353(3) | 14.2(3) s | ß+ to 164Hf | (3+) | N/A | 1289.58 | - |
| 165Ta | 164.950773(19) | 31.0(15) s | ß+ to 165Hf | 5/2-# | N/A | 1297.66 | - |
| 166Ta | 165.95051(3) | 34.4(5) s | ß+ to 166Hf | (2)+ | N/A | 1305.74 | - |
| 167Ta | 166.94809(3) | 1.33(7) min | ß+ to 167Hf | (3/2+) | N/A | 1323.13 | - |
| 168Ta | 167.94805(3) | 2.0(1) min | ß+ to 168Hf | (2-,3+) | N/A | 1331.21 | - |
| 169Ta | 168.94601(3) | 4.9(4) min | ß+ to 169Hf | (5/2+) | N/A | 1339.29 | - |
| 170Ta | 169.94618(3) | 6.76(6) min | ß+ to 170Hf | (3)(+#) | N/A | 1347.37 | - |
| 171Ta | 170.94448(3) | 23.3(3) min | ß+ to 171Hf | (5/2-) | N/A | 1355.45 | - |
| 172Ta | 171.94490(3) | 36.8(3) min | ß+ to 172Hf | (3+) | N/A | 1363.52 | - |
| 173Ta | 172.94375(3) | 3.14(13) h | ß+ to 173Hf | 5/2- | N/A | 1371.6 | - |
| 174Ta | 173.94445(3) | 1.14(8) h | ß+ to 174Hf | 3+ | N/A | 1379.68 | - |
| 175Ta | 174.94374(3) | 10.5(2) h | ß+ to 175Hf | 7/2+ | N/A | 1387.76 | - |
| 176Ta | 175.94486(3) | 8.09(5) h | ß+ to 176Hf | (1)- | N/A | 1395.84 | - |
| 177Ta | 176.944472(4) | 56.56(6) h | EC to 177Hf | 7/2+ | 2.25 | 1403.92 | - |
| 178Ta | 177.945778(16) | 9.31(3) min | EC to 178Hf | 1+ | 2.74 | 1412 | - |
| 179Ta | 178.9459295(23) | 1.82(3) y | EC to 179Hf | 7/2+ | N/A | 1420.08 | - |
| 180Ta | 179.947462 (4) | Observationally Stable | - | 9- | N/A | 1428.15 | - |
| 180mTa | 179.9474648(24) | 8.152(6) h | EC to 180Hf; ß- to 180W | 1+ | N/A | 1444.661743 | 0.012 |
| 181Ta | 180.9479958(19) | Observationally Stable | - | 7/2+ | 2.371 | 1436.23 | 99.988 |
| 182Ta | 181.9501518(19) | 114.43(3) d | ß- to 182W | 3- | 3.02 | 1435 | - |
| 183Ta | 182.9513726(19) | 5.1(1) d | ß- to 183W | 7/2+ | 2.36 | 1443.07 | - |
| 184Ta | 183.954008(28) | 8.7(1) h | ß- to 184W | (5-) | N/A | 1451.15 | - |
| 185Ta | 184.955559(15) | 49.4(15) min | ß- to 185W | (7/2+)# | N/A | 1459.23 | - |
| 186Ta | 185.95855(6) | 10.5(3) min | ß- to 186W | (2-,3-) | N/A | 1467.31 | - |
| 187Ta | 186.96053(21)# | 2# min [>300 ns] | ß- to 187W | 7/2+# | N/A | 1466.07 | - |
| 188Ta | 187.96370(21)# | 20# s [>300 ns] | ß- to 188W | N/A | N/A | 1474.15 | - |
| 189Ta | 188.96583(32)# | 3# s [>300 ns] | Unknown | 7/2+# | N/A | 1482.23 | - |
| 190Ta | 189.96923(43)# | 0.3# s | Unknown | N/A | N/A | 1490.31 | - |