SECTION 1. IDENTIFICATION
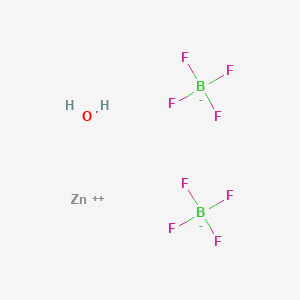
Product Name: Zinc Tetrafluoroborate Hydrate
Product Number: All applicable American Elements product codes, e.g. ZN-FBAT-02-C.XHYD
, ZN-FBAT-03-C.XHYD
, ZN-FBAT-04-C.XHYD
, ZN-FBAT-05-C.XHYD
CAS #: 27860-83-9
Relevant identified uses of the substance: Scientific research and development
Supplier details:
American Elements
10884 Weyburn Ave.
Los Angeles, CA 90024
Tel: +1 310-208-0551
Fax: +1 310-208-0351
Emergency telephone number:
Domestic, North America: +1 800-424-9300
International: +1 703-527-3887
SECTION 2. HAZARDS IDENTIFICATION
2.1 Classification of the substance or mixture
GHS Classification in accordance with 29 CFR 1910 (OSHA HCS)
Acute toxicity, Oral (Category 3), H301
Skin corrosion (Category 1B), H314
Serious eye damage (Category 1), H318
2.2 GHS Label elements, including precautionary statements
Pictogram
Signal word Danger
Hazard statement(s)
H301 Toxic if swallowed.
H314 Causes severe skin burns and eye damage.
Precautionary statement(s)
P260 Do not breathe dusts or mists.
P264 Wash skin thoroughly after handling.
P270 Do not eat, drink or smoke when using this product.
P280 Wear protective gloves/ protective clothing/ eye protection/ face
protection.
P301 + P310 + P330 IF SWALLOWED: Immediately call a POISON CENTER/ doctor.
Rinse mouth.
P301 + P330 + P331 IF SWALLOWED: Rinse mouth. Do NOT induce vomiting.
P303 + P361 + P353 IF ON SKIN (or hair): Take off immediately all contaminated
clothing. Rinse skin with water/ shower.
P304 + P340 + P310 IF INHALED: Remove person to fresh air and keep comfortable
for breathing. Immediately call a POISON CENTER/ doctor.
P305 + P351 + P338 + P310
IF IN EYES: Rinse cautiously with water for several minutes.
Remove contact lenses, if present and easy to do. Continue
rinsing. Immediately call a POISON CENTER/ doctor.
P363 Wash contaminated clothing before reuse.
P405 Store locked up.
P501 Dispose of contents/ container to an approved waste disposal plant.
2.3 Hazards not otherwise classified (HNOC) or not covered by GHS
Weak hydrogen fluoride-releaser


SECTION 3. COMPOSITION/INFORMATION ON INGREDIENTS
3.1 Substances
Formula : B2F8Zn · xH2O
Molecular weight : 239.00 g/mol
CAS-No. : 27860-83-9
EC-No. : 237-534-0
SECTION 4. FIRST AID MEASURES
4.1 Description of first-aid measures
General advice
Hydrofluoric (HF) acid burns require immediate and specialized first aid and medical
treatment. Symptoms may be delayed up to 24 hours depending on the concentration of
HF. After decontamination with water, further damage can occur due to
penetration/absorption of the fluoride ion. Treatment should be directed toward binding the
fluoride ion as well as the effects of exposure. Skin exposures can be treated with a 2.5%
calcium gluconate gel repeated until burning ceases. More serious skin exposures may
require subcutaneous calcium gluconate except for digital areas unless the physician is
experienced in this technique, due to the potential for tissue injury from increased
pressure. Absorption can readily occur through the subungual areas and should be
considered when undergoing decontamination. Prevention of absorption of the fluoride ion
in cases of ingestion can be obtained by giving milk, chewable calcium carbonate tablets or
Milk of Magnesia to conscious victims. Conditions such as hypocalcemia, hypomagnesemia
and cardiac arrhythmias should be monitored for, since they can occur after exposure.First
aiders need to protect themselves. Show this material safety data sheet to the doctor in
attendance.
If inhaled
After inhalation: fresh air. Call in physician.
In case of skin contact
First treatment with calcium gluconate paste.In case of skin contact: Take off immediately
all contaminated clothing. Rinse skin with water/ shower. Call a physician immediately.
In case of eye contact
After eye contact: rinse out with plenty of water. Immediately call in ophthalmologist.
Remove contact lenses.
If swallowed
If swallowed: give water to drink (two glasses at most). Seek medical advice immediately.
In exceptional cases only, if medical care is not available within one hour, induce vomiting
(only in persons who are wide awake and fully conscious), administer activated charcoal
(20 - 40 g in a 10% slurry) and consult a doctor as quickly as possible. Do not attempt to
neutralise.
4.2 Most important symptoms and effects, both acute and delayed
The most important known symptoms and effects are described in the labelling (see section
2.2) and/or in section 11
4.3 Indication of any im
SECTION 5. FIREFIGHTING MEASURES
5.1 Extinguishing media
Suitable extinguishing media
Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide.Use extinguishing
measures that are appropriate to local circumstances and the surrounding environment.
Unsuitable extinguishing media
For this substance/mixture no limitations of extinguishing agents are given.For this
substance/mixture no limitations of extinguishing agents are given.
5.2 Special hazards arising from the substance or mixture
Hydrogen fluoride
Borane/boron oxides
Zinc/zinc oxides
Not combustible.
Ambient fire may liberate hazardous vapours.
5.3 Advice for firefighters
Stay in danger area only with self-contained breathing apparatus. Prevent skin contact by
keeping a safe distance or by wearing suitable protective clothing.
5.4 Further information
Suppress (knock down) gases/vapors/mists with a water spray jet. Prevent fire
extinguishing water from contaminating surface water or the ground water system.
SECTION 6. ACCIDENTAL RELEASE MEASURES
6.1 Personal precautions, protective equipment and emergency procedures
Advice for non-emergency personnel: Avoid inhalation of dusts. Avoid substance contact.
Ensure adequate ventilation. Evacuate the danger area, observe emergency procedures,
consult an expert.
For personal protection see section 8.
6.2 Environmental precautions
Do not let product enter drains.
6.3 Methods and materials for containment and cleaning up
Cover drains. Collect, bind, and pump off spills. Observe possible material restrictions
(see sections 7 and 10). Take up carefully. Dispose of properly. Clean up affected area.
Avoid generation of dusts.
6.4 Reference to other sections
For disposal see section 13.
SECTION 7. HANDLING AND STORAGE
7.1 Precautions for safe handling
For precautions see section 2.2.
7.2 Conditions for safe storage, including any incompatibilities
Storage conditions
Tightly closed. Dry. Keep in a well-ventilated place. Keep locked up or in an area accessible
only to qualified or authorized persons.
Storage class
Storage class (TRGS 510): 6.1D: Non-combustible, acute toxic Cat.3 / toxic hazardous
materials or hazardous materials causing chronic effects
7.3 Specific end use(s)
Apart from the uses mentioned in section 1.2 no other specific uses are stipulated
SECTION 8. EXPOSURE CONTROLS/PERSONAL PROTECTION
8.1 Control parameters
Ingredients with workplace control parameters
Contains no substances with occupational exposure limit values.
8.2 Exposure controls
Appropriate engineering controls
Immediately change contaminated clothing. Apply preventive skin protection. Wash
hands and face after working with substance.
Personal protective equipment
Eye/face protection
Use equipment for eye protection tested and approved under appropriate
government standards such as NIOSH (US) or EN 166(EU). Tightly fitting safety
goggles
Skin protection
Handle with impervious gloves.
This recommendation applies only to the product stated in the safety data sheet,
supplied by us and for the designated use. When dissolving in or mixing with other
substances and under conditions deviating from those stated in EN374 please
contact the supplier of CE-approved gloves (e.g. KCL GmbH, D-36124 Eichenzell,
Internet: www.kcl.de).
Full contact
Material: Nitrile rubber
Minimum layer thickness: 0.11 mm
Break through time: 480 min
Material tested:KCL 741 Dermatril® L
Splash contact
Material: Nitrile rubber
Minimum layer thickness: 0.11 mm
Break through time: 480 min
Material tested:KCL 741 Dermatril® L
Body Protection
protective clothing
Respiratory protection
required when dusts are generated.
Our recommendations on filtering respiratory protection are based on the following
standards: DIN EN 143, DIN 14387 and other accompanying standards relating to
the used respiratory protection system.
Control of environmental exposure
Do not let product enter drains.
SECTION 9. PHYSICAL AND CHEMICAL PROPERTIES
9.1 Information on basic physical and chemical properties
a) Appearance Form: crystalline
Color: white
b) Odor No data available
c) Odor Threshold No data available
d) pH No data available
e) Melting
point/freezing point
No data available
f) Initial boiling point
and boiling range
No data available
g) Flash point Not applicable
h) Evaporation rate No data available
i) Flammability (solid,
gas)
The product is not flammable.
j) Upper/lower
flammability or
explosive limits
No data available
k) Vapor pressure No data available
l) Vapor density No data available
m) Density No data available
Relative density No data available
n) Water solubility No data available
o) Partition coefficient:
n-octanol/water
No data available
p) Autoignition
temperature
No data available
q) Decomposition
temperature
No data available
r) Viscosity No data available
s) Explosive properties No data available
t) Oxidizing properties none
9.2 Other safety information
No data available
SECTION 10. STABILITY AND REACTIVITY
10.1 Reactivity
No data available
10.2 Chemical stability
The product is chemically stable under standard ambient conditions (room temperature) .
10.3 Possibility of hazardous reactions
No data available
10.4 Conditions to avoid
no information available
10.5 Incompatible materials
Strong oxidizing agents
10.6 Hazardous decomposition products
In the event of fire: see section 5
SECTION 11. TOXICOLOGICAL INFORMATION
11.1 Information on toxicological effects
Acute toxicity
Oral: No data available
Inhalation: No data available
Dermal: No data available
No data available
Skin corrosion/irritation
No data available
Serious eye damage/eye irritation
No data available
Respiratory or skin sensitization
No data available
Germ cell mutagenicity
No data available
Carcinogenicity
IARC: No ingredient of this product present at levels greater than or equal to 0.1% is
identified as probable, possible or confirmed human carcinogen by IARC.
NTP: No ingredient of this product present at levels greater than or equal to 0.1% is
identified as a known or anticipated carcinogen by NTP.
OSHA: No component of this product present at levels greater than or equal to 0.1% is
on OSHA’s list of regulated carcinogens.
Reproductive toxicity
No data available
Specific target organ toxicity - single exposure
No data available
Specific target organ toxicity - repeated exposure
No data available
Aspiration hazard
No data available
11.2 Additional Information
Fluoride ion can reduce serum calcium levels possibly causing fatal hypocalcemia.
Material is extremely destructive to tissue of the mucous membranes and upper respiratory
tract, eyes, and skin., spasm, inflammation and edema of the larynx, spasm, inflammation
and edema of the bronchi, pneumonitis, pulmonary edema, burning sensation, Cough,
wheezing, laryngitis, Shortness of breath, Headache, Nausea
To the best of our knowledge, the chemical, physical, and toxicological properties have not
been thoroughly investigated.
SECTION 12. ECOLOGICAL INFORMATION
12.1 Toxicity
No data available
12.2 Persistence and degradability
No data available
12.3 Bioaccumulative potential
No data available
12.4 Mobility in soil
No data available
12.5 Results of PBT and vPvB assessment
PBT/vPvB assessment not available as chemical safety assessment not required/not
conducted
12.6 Endocrine disrupting properties
No data available
12.7 Other adverse effects
No data available
SECTION 13. DISPOSAL CONSIDERATIONS
13.1 Waste treatment methods
Product
Waste material must be disposed of in accordance with the national and local regulations.
Leave chemicals in original containers. No mixing with other waste. Handle uncleaned
containers like the product itself. See www.retrologistik.com for processes regarding the
return of chemicals and containers, or contact us there if you have further questions.
SECTION 14. TRANSPORT INFORMATION
DOT (US)
UN number: 3260 Class: 8 Packing group: II
Proper shipping name: Corrosive solid, acidic, inorganic, n.o.s. (Zinc tetrafluoroborate
hydrate)
Reportable Quantity (RQ):
Poison Inhalation Hazard: No
IMDG
UN number: 3260 Class: 8 Packing group: II EMS-No: F-A, S-B
Proper shipping name: CORROSIVE SOLID, ACIDIC, INORGANIC, N.O.S. (Zinc
tetrafluoroborate hydrate)
IATA
UN number: 3260 Class: 8 Packing group: II
Proper shipping name: Corrosive solid, acidic, inorganic, n.o.s. (Zinc tetrafluoroborate
hydrate)
SECTION 15. REGULATORY INFORMATION
SARA 302 Components
This material does not contain any components with a section 302 EHS TPQ.
SARA 313 Components
The following components are subject to reporting levels established by SARA Title III,
Section 313:
Zinc tetrafluoroborate hydrate
CAS-No.
27860-83-9
Revision Date
2015-07-08
SARA 311/312 Hazards
Acute Health Hazard
Massachusetts Right To Know Components
No components are subject to the Massachusetts Right to Know Act.
SECTION 16. OTHER INFORMATION
Safety Data Sheet according to Regulation (EC) No. 1907/2006 (REACH). The above information is believed to be correct but does not purport to be all inclusive and shall be used only as a guide. The information in this document is based on the present state of our knowledge and is applicable to the product with regard to appropriate safety precautions. It does not represent any guarantee of the properties of the product. American Elements shall not be held liable for any damage resulting from handling or from contact with the above product. See reverse side of invoice or packing slip for additional terms and conditions of sale. COPYRIGHT 1997-2022 AMERICAN ELEMENTS. LICENSED GRANTED TO MAKE UNLIMITED PAPER COPIES FOR INTERNAL USE ONLY.
 Boron (atomic symbol: B, atomic number: 5) is a Block P, Group 13, Period 2 element with an atomic weight of 10.81. The number of electrons in each of boron's shells is 2, 3 and its electron configuration is [He] 2s2 2p1. The boron atom has a radius of 90 pm and a Van der Waals radius of 192 pm. Boron was discovered by Joseph Louis Gay-Lussac and Louis Jacques Thénard in 1808 and was first isolated by Humphry Davy later that year. Boron is classified as a metalloid is not found naturally on earth.
Boron (atomic symbol: B, atomic number: 5) is a Block P, Group 13, Period 2 element with an atomic weight of 10.81. The number of electrons in each of boron's shells is 2, 3 and its electron configuration is [He] 2s2 2p1. The boron atom has a radius of 90 pm and a Van der Waals radius of 192 pm. Boron was discovered by Joseph Louis Gay-Lussac and Louis Jacques Thénard in 1808 and was first isolated by Humphry Davy later that year. Boron is classified as a metalloid is not found naturally on earth.  Along with carbon and nitrogen, boron is one of the few elements in the periodic table known to form stable
Along with carbon and nitrogen, boron is one of the few elements in the periodic table known to form stable  The zinc atom has a radius of 134 pm and a Van der Waals radius of 210 pm. Zinc was discovered by Indian
The zinc atom has a radius of 134 pm and a Van der Waals radius of 210 pm. Zinc was discovered by Indian  It is a fair conductor of electricity, and burns in air at high red producing white clouds of the
It is a fair conductor of electricity, and burns in air at high red producing white clouds of the