About Silver
As one of the first precious metals known by man, silver has always been a highly valued material for coinage, jewelry, and decorative objects. Based on its properties, the metal also has wide-reaching applications in modern technology, but its relative high cost often encourages the use of cheaper alternative materials. Yet the existence of easily accessible silver stockpiles have led to its substitution for cheaper metals that are temporarily in short supply. Most notably, silver reserves were tapped during the world wars, substituting for copper in electrical applications, tin in solder, and nickel in the production of coins.
Silver has the highest electrical conductivity of any element. Copper is a more economically viable substitute, but for some applications the energy savings or performance benefits provided by the substitution of silver are substantial enough to overcome this cost barrier. Silver may be used for electronics applications in cases where other key physical or chemical properties are uniquely advantageous: for instance, silver nanowires can be used to produce transparent and flexible electrodes for use in photovoltaic solar cells, and silver nanoparticles and conductive silver inks are used in the production of RFID tags and membrane switches used for TV remote controls, computer keyboards, and control panels on home appliances. Silver cadmium oxide and other conductive silver compounds are favored for use in high-voltage contacts because they resist the effect of electrical arcing. Silver also is a component of some materials used for phase-change memory technologies such as rewritable optical discs (CD-RW).
Mirrors can be produced by a chemical process that coats glass with thin layer of silver metal, often termed silvering, which was discovered in 1835. Today, standard mirrors are usually produced using sputtered thin coats of aluminum, as it is cheaper than silver and less subject to tarnishing, but glass ornaments and some high quality mirrors are still sometimes made with the silvering process. Thinner layers of silver are visually transparent but effectively block UV radiation; today optical glass coated with such layers is used in energy-saving window panes. Film photography was first developed around the same time, and then as now, silver was an essential component of almost all film photography processes, which exploit the photosensitivity of silver halide compounds. Finally, silver ions are naturally germicidal, and silver and its compounds have been used in wound care and as disinfectant for centuries. Today, silver is still found in antimicrobial creams for treating burns, and silver nanoparticles are used in water filtration systems and embedded in clothing to deter bacterial growth.
Several specialized battery formulations contain silver salts. Silver oxide batteries have a long working life and high energy-to-weight ratio, and therefore are used in small devices such as hearing aids. Silver-zinc batteries are likewise valued for high-energy density, as well as for being extremely safe and reliable, with a long life both on the shelf and in active use. Silver-zinc formulations are frequently used in batteries designed for aerospace and defense applications such as NASA launch vehicles, missiles, and satellites. As with the use of silver as a conductor for electronics, the use of these batteries are limited by the added expense of silver, but in some applications the advantages are considered worth the cost.
An additional key use for silver is in silver soldering and brazing. Both are methods used to join metallic components, but they vary in the composition of the joining material and the temperatures required. Silver soldering is a lower temperature process often used in jewelry making or as a substitute for lead-based solders, and often uses tin-silver or tin-silver-copper formulations. Silver brazing is a higher temperature process that produces an extremely strong joint that will resist significant shock and vibration while using very small amounts of a silver brazing alloy. Brazing is used frequently for attaching cemented carbide tips to tools.
Silver staining procedures, which typically make use of soluble silver nitrate along with various sensitizers and fixatives, are used in biology labs, as silver ions bind tightly to most proteins and allow their visualization on diagnostic gels, in karyotypes, and in tissue samples. Silver is often included in nuclear control rods to absorb free neutrons and used to plate steel bearings for use in automotive or jet engines to reduce friction. Amalgam fillings made of mercury in combination with silver or gold are still used in dentistry, but increasingly ceramic composites are favored for cosmetic reasons and due to safety concerns related to the use of mercury. Silver also serves as a catalyst in the industrial production of ethylene oxide and formaldehyde or as one of several catalysts found in catalytic converters, but platinum group metals are usually preferred for this purpose.
Silver can be found as a native metal or as part of natural alloys with gold, but is more often a component of minerals such as argentite. It is most often produced for commercial uses as a byproduct of mining and refining copper, copper-nickel, lead, and lead-zinc ores, which frequently contain some amount of silver minerals.
Products
 Silver has a long history as a precious metal, having been used in coins, ornaments, jewelry, and utensils (silverware). Silver is also used for electrical contacts and conductors. Silver compounds are sometimes used as catalyst of chemical reactions. Silver nitrate has wide application in painting, xerography, chemical electroplating and electric batteries. Silver chloride is another important compound, due to its ductility and malleability.
Silver has a long history as a precious metal, having been used in coins, ornaments, jewelry, and utensils (silverware). Silver is also used for electrical contacts and conductors. Silver compounds are sometimes used as catalyst of chemical reactions. Silver nitrate has wide application in painting, xerography, chemical electroplating and electric batteries. Silver chloride is another important compound, due to its ductility and malleability.  The organic compounds of the element are used in the coating of several metals and in dynamite or other explosive bars. Silver is available as metal and compounds with purities from 99% to 99.999% (ACS grade to ultra-high purity). Elemental or metallic forms include pellets, rod, wire and granules for evaporation source material purposes. Silver nanoparticles and nanopowders provide ultra-high surface area. Oxides are available in powder and dense pellet form for such uses as optical coating and thin film applications. Oxides tend to be insoluble. Fluorides are another insoluble form for uses in which oxygen is undesirable such as metallurgy, chemical and physical vapor deposition and in some optical coatings. Silver is also available in soluble forms including chlorides, nitrates and acetates. These compounds can be manufactured as solutions at specified stoichiometries.
The organic compounds of the element are used in the coating of several metals and in dynamite or other explosive bars. Silver is available as metal and compounds with purities from 99% to 99.999% (ACS grade to ultra-high purity). Elemental or metallic forms include pellets, rod, wire and granules for evaporation source material purposes. Silver nanoparticles and nanopowders provide ultra-high surface area. Oxides are available in powder and dense pellet form for such uses as optical coating and thin film applications. Oxides tend to be insoluble. Fluorides are another insoluble form for uses in which oxygen is undesirable such as metallurgy, chemical and physical vapor deposition and in some optical coatings. Silver is also available in soluble forms including chlorides, nitrates and acetates. These compounds can be manufactured as solutions at specified stoichiometries.
Silver Properties
 Silver is a Block D, Group 11, Period 5 element.
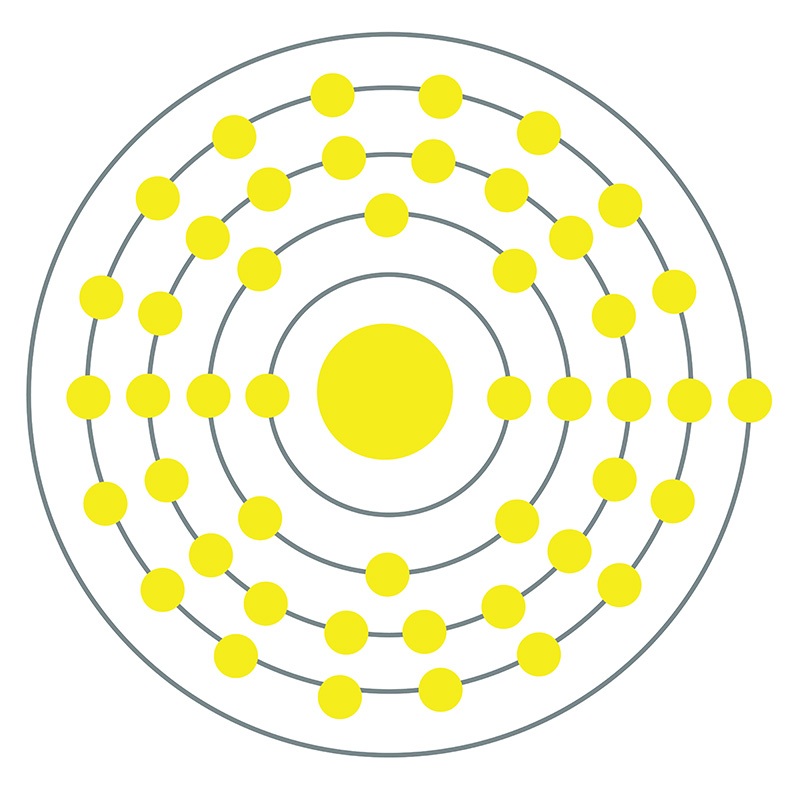
Silver is a Block D, Group 11, Period 5 element. 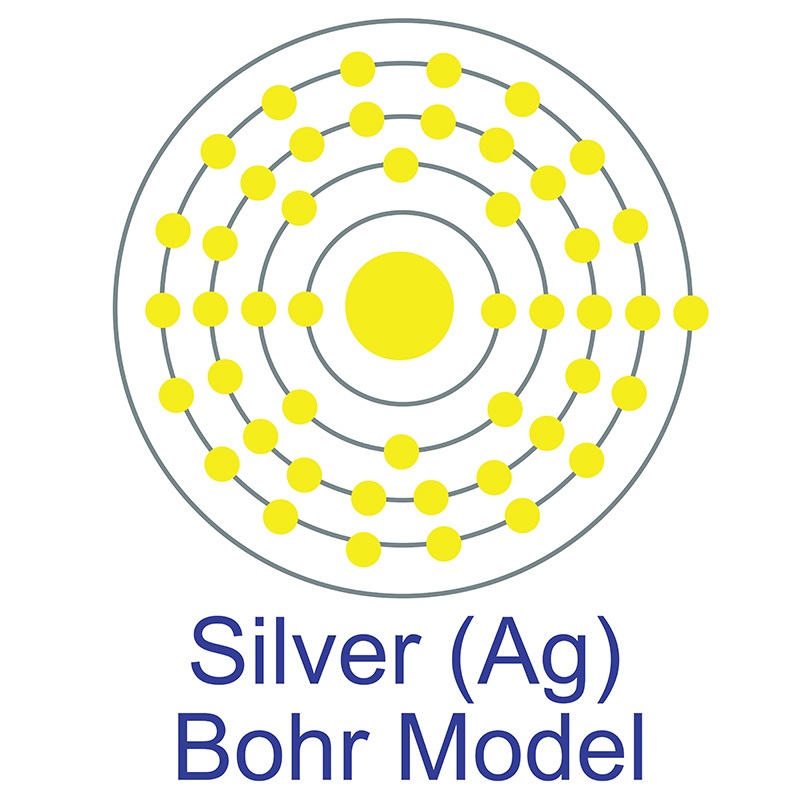 The number of electrons in each of Silver's shells is 2, 8, 18, 18, 1 and its electron configuration is [Kr]4d10 5s1.
The number of electrons in each of Silver's shells is 2, 8, 18, 18, 1 and its electron configuration is [Kr]4d10 5s1.  The silver atom has a radius of 144.5.pm and its Van der Waals radius is 144.pm. In its elemental form, CAS 7440-22-4, silver has a brilliant white metallic luster. It is a little harder than gold and is very ductile and malleable, being exceeded only by gold and perhaps palladium. Pure silver has the highest electrical and thermal conductivity of all metals, and possesses the lowest contact resistance. It is stable in pure air and water, but tarnishes when exposed to ozone, hydrogen sulfide, or air containing sulfur. Silver was first used by early humans, begininng prior to 5000 BC. It is found in copper, copper-nickel, lead, and lead-zinc ores among others. Silver was named after the Anglo-Saxon word "seolfor" or "siolfur" meaning 'silver'.
The silver atom has a radius of 144.5.pm and its Van der Waals radius is 144.pm. In its elemental form, CAS 7440-22-4, silver has a brilliant white metallic luster. It is a little harder than gold and is very ductile and malleable, being exceeded only by gold and perhaps palladium. Pure silver has the highest electrical and thermal conductivity of all metals, and possesses the lowest contact resistance. It is stable in pure air and water, but tarnishes when exposed to ozone, hydrogen sulfide, or air containing sulfur. Silver was first used by early humans, begininng prior to 5000 BC. It is found in copper, copper-nickel, lead, and lead-zinc ores among others. Silver was named after the Anglo-Saxon word "seolfor" or "siolfur" meaning 'silver'.
Health, Safety & Transportation Information for Silver
Silver is not toxic although most silver salts are poisonous. Safety data for Silver and its compounds can vary widely depending on the form. For potential hazard information, toxicity, and road, sea and air transportation limitations, such as DOT Hazard Class, DOT Number, EU Number, NFPA Health rating and RTECS Class, please see the specific material or compound referenced in the Products tab. The below information applies to elemental (metallic) Silver.
| Safety Data | |
|---|---|
| Signal Word | N/A |
| Hazard Statements | N/A |
| Hazard Codes | N/A |
| Risk Codes | N/A |
| Safety Precautions | N/A |
| RTECS Number | N/A |
| Transport Information | N/A |
| WGK Germany | 3 |
| Globally Harmonized System of Classification and Labelling (GHS) |
N/A |
Silver Isotopes
Naturally occurring silver (Ag) has two stable isotopes: 107Ag and 109Ag.
| Nuclide | Isotopic Mass | Half-Life | Mode of Decay | Nuclear Spin | Magnetic Moment | Binding Energy (MeV) | Natural Abundance (% by atom) |
|---|---|---|---|---|---|---|---|
| 93Ag | 92.94978(64)# | 5# ms [>1.5 µs] | Unknown | 9/2+# | N/A | 746.1 | - |
| 94Ag | 93.94278(54)# | 37(18) ms [26(+26-9) ms] | ß+ to 94Pd | 0+# | N/A | 760.7 | - |
| 95Ag | 94.93548(43)# | 1.74(13) s | ß+ to 95Pd; ß+ + p to 94Pd | (9/2+) | N/A | 775.3 | - |
| 96Ag | 95.93068(43)# | 4.45(4) s | ß+ to 96Pd; ß+ + p to 95Pd | (8+) | N/A | 788.03 | - |
| 97Ag | 96.92397(35) | 25.3(3) s | ß+ to 97Pd | (9/2+) | N/A | 802.63 | - |
| 98Ag | 97.92157(7) | 47.5(3) s | ß+ to 98Pd; ß+ + p to 97Pd | (5+) | N/A | 812.58 | - |
| 99Ag | 98.91760(16) | 124(3) s | ß+ to 99Pd | (9/2)+ | N/A | 824.38 | - |
| 100Ag | 99.91610(8) | 2.01(9) min | ß+ to 100Pd | (5)+ | N/A | 833.39 | - |
| 101Ag | 100.91280(11) | 11.1(3) min | ß+ to 101Pd | 9/2+ | N/A | 847.06 | - |
| 102Ag | 101.91169(3) | 12.9(3) min | ß+ to 102Pd | 5+ | N/A | 855.14 | - |
| 103Ag | 102.908973(18) | 65.7(7) min | EC to 103Pd | 7/2+ | 4.47 | 872.53 | - |
| 104Ag | 103.908629(6) | 69.2(10) min | EC to 104Pd | 5+ | 3.92 | 880.61 | - |
| 105Ag | 104.906529(12) | 41.29(7) d | EC to 105Pd | 1/2- | 0.1014 | 888.69 | - |
| 106Ag | 105.906669(5) | 23.96(4) min | EC to 106Pd; ß- to 106Cd | 1+ | 3.71 | 896.77 | 51.839 |
| 107Ag | 106.905097(5) | STABLE | - | 1/2- | -0.11357 | 904.85 | - |
| 108Ag | 107.905956(5) | 2.37(1) min | EC to 108Pd; ß- to 108Cd | 1+ | 2.6884 | 912.93 | 48.161 |
| 109Ag | 108.904752(3) | STABLE | - | 1/2- | -0.1306905 | 921.01 | - |
| 110Ag | 109.906107(3) | 24.6(2) s | EC to 110Pd; ß- to 110Cd | 1+ | 2.7271 | 929.08 | - |
| 111Ag | 110.905291(3) | 7.45(1) d | ß- to 111Cd | 1/2- | N/A | 937.16 | - |
| 112Ag | 111.907005(18) | 3.130(9) h | ß- to 112Cd | 2(-) | 0.0547 | 945.24 | - |
| 113Ag | 112.906567(18) | 5.37(5) h | ß- to 113Cd | 1/2- | 0.159 | 953.32 | - |
| 114Ag | 113.908804(27) | 4.6(1) s | ß- to 114Cd | 1+ | N/A | 961.4 | - |
| 115Ag | 114.90876(4) | 20.0(5) min | ß- to 115Cd | 1/2- | N/A | 969.48 | - |
| 116Ag | 115.91136(5) | 2.68(10) min | ß- to 116Cd | (2)- | N/A | 968.24 | - |
| 117Ag | 116.91168(5) | 73.6(14) s [72.8(+20-7) s] | ß- to 117Cd | 1/2-# | N/A | 976.32 | - |
| 118Ag | 117.91458(7) | 3.76(15) s | ß- to 118Cd | 1- | N/A | 984.4 | - |
| 119Ag | 118.91567(10) | 6.0(5) s | ß- to 119Cd | 1/2-# | N/A | 992.48 | - |
| 120Ag | 119.91879(8) | 1.23(4) s | ß- to 120Cd; ß- + n to 119Cd | 3(+#) | N/A | 1000.55 | - |
| 121Ag | 120.91985(16) | 0.79(2) s | ß- to 121Cd; ß- + n to 120Cd | (7/2+)# | N/A | 1008.63 | - |
| 122Ag | 121.92353(22)# | 0.529(13) s | ß- to 122Cd; ß- + n to 121Cd | (3+) | N/A | 1007.4 | - |
| 123Ag | 122.92490(22)# | 0.300(5) s | ß- to 123Cd; ß- + n to 122Cd | (7/2+) | N/A | 1015.47 | - |
| 124Ag | 123.92864(21)# | 172(5) ms | ß- to 124Cd; ß- + n to 123Cd | 3+# | N/A | 1023.55 | - |
| 125Ag | 124.93043(32)# | 166(7) ms | ß- to 125Cd; ß- + n to 124Cd | (7/2+)# | N/A | 1022.32 | - |
| 126Ag | 125.93450(32)# | 107(12) ms | ß- to 126Cd; ß- + n to 125Cd | 3+# | N/A | 1030.39 | - |
| 127Ag | 126.93677(32)# | 79(3) ms | ß- to 127Cd; ß- + n to 126Cd | 7/2+# | N/A | 1038.47 | - |
| 128Ag | 127.94117(32)# | 58(5) ms | Unknown | N/A | N/A | 1037.24 | - |
| 129Ag | 128.94369(43)# | 44(7) ms [46(+5-9) ms] | Unknown | 7/2+# | N/A | 1045.31 | - |
| 130Ag | 129.95045(36)# | ~50 ms | Unknown | 0+ | N/A | 1044.08 | - |