About Sulfur
Because it is abundant and is often found in elemental form, sulfur has been known and used widely for most of human history. In fact, the alchemists regarded sulfur as one of the three basic substances of which everything in the universe was composed. The word “sulfur” comes from a latin root meaning “to burn”, and historically was sometimes used broadly to describe flammable substances. Some of sulfur’s oldest recorded uses were in traditional medicine, fumigation, and bleaching cloths. Later, it was produced in large quantities for use in gunpowder. In the early days of chemistry, popular opinion held that sulfur was a compound, but in 1777 Antoine Lavoisier, often described as a father of modern chemistry, made a compelling case that it was in fact an element, which soon became the dominant view.
The largest use of sulfur is in the production of sulfuric acid, a chemical so essential to modern industry that its consumption is considered one of the best indicators of a nation’s industrial development. In industrial chemistry, it is used in the production of ammonia, aluminum hydroxide, phosphate fertilizers, alums, dyes, and refined petroleum products. It also serves as an acid catalyst and an industrial cleaning agent. Additionally, it is a major component of lead-acid batteries found in most automobiles, as well as some household drain cleaners. It is a component of the sulfur-iodine cycle, a process that has been proposed for production of hydrogen as fuel.
Many other forms of sulfur are important chemical reagents. Elemental sulfur is a necessary component of vulcanized rubber, which is stabilized by disulfide bond crosslinking. Carbon disulfide is an important organic chemistry reagent, used as an industrial non-polar solvent and a key player in the manufacture of polymers such as cellophane and rayon. Sulfur dioxide can be used as a bleaching agents for paper and delicate fabrics, and in wastewater treatment to remove chlorine. Many sulfur compounds, including the common sodium lauryl sulfate, serve as detergents and surfactants, found in everything from personal care products such as shampoo to lab reagents used in molecular biology. Hydrogen sulfide is an important intermediate used to produce metal sulfides and organosulfur components. Sulfur hexafluoride is a gaseous dielectric used in high-voltage circuit breakers and switchgear, as well as in the high-voltage power supplies for scientific equipment such as particle accelerators and electron microscopes. It is also used as an etchant in semiconductor manufacturing, a tracer compound of use for studying spread of toxic agents through the air. Phosphorus sesquisulfide is used to produce “strike anywhere” matches, while other sulfides are used in the production of safety matches.
Elemental sulfur is not toxic to humans, but it can be used as a pesticide. Additionally, many sulfur compounds are used for their toxicity to microbes. Sulfur compounds have long been used in winemaking to prevent the souring caused by bacterial growth during fermentation, and sulfites are often used to preserve food, particularly dried fruit and molasses. Gaseous sulfur compounds that do exhibit toxicity to humans have a history of use in warfare; mustard gas is one example.
Sulfur compounds also play an important role in the technology industry. Several types of lithium ion batteries utilize sulfur compounds, and sodium-sulfur batteries are used in large-scale energy storage applications. Additionally, many sulfide compounds are semiconductors that are either in active use or are the subject of current investigation. Cadmium sulfide is used primarily as a pigment, but can also be used in photoresistors, thin film transistors, and semiconductor lasers. Iron pyrite is abundant, cheap and had favorable chemistry for use in solar cells, though fabrication of actual cells has faced many challenges. Copper zinc tin sulfide is also of interest for use in photovoltaics, particularly in thin film device designs. Molybdenum disulfide, like the nanomaterial graphene, can be grown in layers a single atom thick and is of interest for use in many optoelectronic applications.
As the tenth most abundant element in the universe, sulfur is everywhere. In addition to being found in native deposits, often in regions with volcanic activity, sulfur is a component of sulfide and sulfate minerals, including many which serve as primary ores for the metals they also contain. Some of these minerals are used directly--for instance gypsum, a natural mineral form of calcium sulfate, is both mined directly and recovered as byproduct of other industrial processes for use as a major component of drywall, plaster, and cement. Elemental sulfur may be extracted from many of these minerals or from pure deposits, but today most industrial sulfur production is a side product of other processes, especially the refining of petroleum products, which in their unprocessed form often contain significant amounts of organosulfur compounds.
Products

Sulfur is a crucial element for all life and serves as both fuel and respiratory (oxygen-replacing) material for simple organisms. As a component of the amino acids methionine and cysteine, sulfur is a component of proteins, and sulfur is present in the vitamins thiamine and biotin. Sulfur is available in elemental and compound forms with purities from 99% to 99.999% (ACS grade to ultra-high purity).
Sulfur Properties
![]() Sulfur is a Block P, Group 16, Period 3 element.
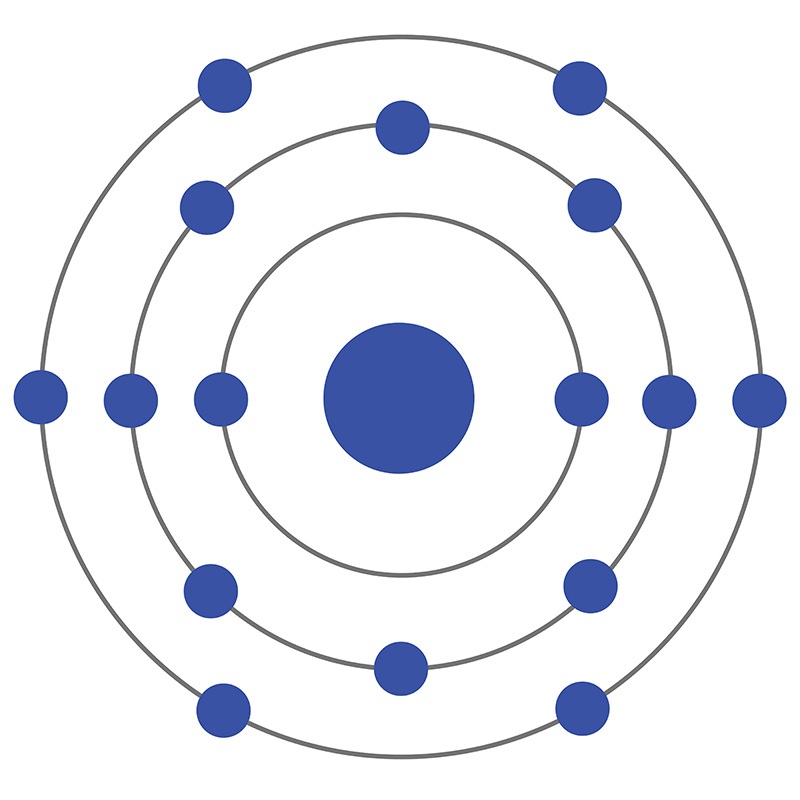
Sulfur is a Block P, Group 16, Period 3 element. 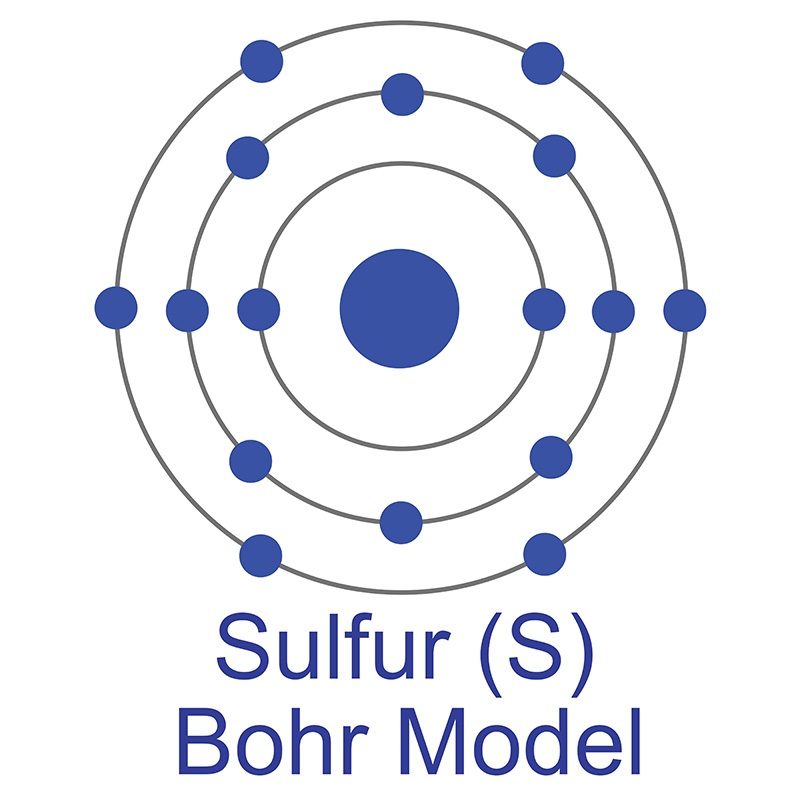 The number of electrons in each of Sulfur's shells is 2, 8, 6 and its electron configuration is [Ne]3s23p4. In its elemental form, CAS 7704-34-9, sulfur has a light yellow appearance. The sulfur atom has a covalent radius of 105.pm and its Van der Waals radius is 180.pm. In nature, sulfur can be found in hot springs, meteorites, volcanoes, and as galena, gypsum, epsom salts. Sulfur has been known since ancient times but was not accepted as an element until 1777 when Antoine Lavoisier helped to convince the scientific community that sulfur was an element and not a compound.
The number of electrons in each of Sulfur's shells is 2, 8, 6 and its electron configuration is [Ne]3s23p4. In its elemental form, CAS 7704-34-9, sulfur has a light yellow appearance. The sulfur atom has a covalent radius of 105.pm and its Van der Waals radius is 180.pm. In nature, sulfur can be found in hot springs, meteorites, volcanoes, and as galena, gypsum, epsom salts. Sulfur has been known since ancient times but was not accepted as an element until 1777 when Antoine Lavoisier helped to convince the scientific community that sulfur was an element and not a compound.
Health, Safety & Transportation Information for Sulfur
Safety data for Sulfur and its compounds can vary widely depending on the form. For potential hazard information, toxicity, and road, sea and air transportation limitations, such as DOT Hazard Class, DOT Number, EU Number, NFPA Health rating and RTECS Class, please see the specific material or compound referenced in the Products tab. The below information applies to nanoscale sulfur (Sulfur Nanoparticles).
| Safety Data | |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315 |
| Hazard Codes | Xi |
| Risk Codes | 38 |
| Safety Precautions | 46 |
| RTECS Number | N/A |
| Transport Information | UN 1350 4.1/PG 3 |
| WGK Germany | 1 |
| Globally Harmonized System of Classification and Labelling (GHS) |
|
Sulfur Isotopes
Sulfur (S) has four stable isotopes: 32S (95.02%), 33S (0.75%), 34S (4.21%), and 36S (0.02%).
| Nuclide | Isotopic Mass | Half-Life | Mode of Decay | Nuclear Spin | Magnetic Moment | Binding Energy (MeV) | Natural Abundance (% by atom) |
|---|---|---|---|---|---|---|---|
| 26S | 26.02788(32)# | 10# ms | 2p to 24Si | 0+ | N/A | 166.94 | - |
| 27S | 27.01883(22)# | 15.5(15) ms | ß+ to 27P; ß+ + 2p to 25Al; ß+ + p to 26Si | (5/2+) | N/A | 183.4 | - |
| 28S | 28.00437(17) | 125(10) ms | ß+ to 28P; ß+ + p to 27Si | 0+ | N/A | 204.52 | - |
| 29S | 28.99661(5) | 187(4) ms | ß+ to 29P; ß+ + p to 28Si | 5/2+ | N/A | 220.06 | - |
| 30S | 29.984903(3) | 1.178(5) s | EC to 30P | 0+ | N/A | 239.31 | - |
| 31S | 30.9795547(16) | 2.572(13) s | EC to 31P | 1/2+ | N/A | 252.05 | - |
| 32S | 31.97207100(15) | STABLE | - | 0+ | 0 | 266.65 | 94.93 |
| 33S | 32.97145876(15) | STABLE | - | 3/2+ | 0.643821 | 275.66 | 0.76 |
| 34S | 33.96786690(12) | STABLE | - | 0+ | 0 | 287.47 | 4.29 |
| 35S | 34.96903216(11) | 87.51(12) d | ß- to 35Cl | 3/2+ | 1 | 293.68 | - |
| 36S | 35.96708076(20) | STABLE | - | 0+ | 0 | 303.62 | 0.02 |
| 37S | 36.97112557(21) | 5.05(2) min | ß- to 37Cl | 7/2- | N/A | 307.98 | - |
| 38S | 37.971163(8) | 170.3(7) min | ß- to 38Cl | 0+ | N/A | 316.06 | - |
| 39S | 38.97513(5) | 11.5(5) s | ß- to 39Cl | (3/2,5/2,7/2)- | N/A | 320.41 | - |
| 40S | 39.97545(15) | 8.8(22) s | ß- to 40Cl | 0+ | N/A | 328.49 | - |
| 41S | 40.97958(13) | 1.99(5) s | ß- to 41Cl; ß- + n to 40Cl | (7/2-)# | N/A | 332.84 | - |
| 42S | 41.98102(13) | 1.013(15) s | ß- to 42Cl; ß- + n to 41Cl | 0+ | N/A | 339.05 | - |
| 43S | 42.98715(22) | 260(15) ms | ß- to 43Cl; ß- + n to 42Cl | 3/2-# | N/A | 341.54 | - |
| 44S | 43.99021(42) | 100(1) ms | ß- to 43Cl; ß- + n to 43Cl | 0+ | N/A | 346.83 | - |
| 45S | 44.99651(187) | 68(2) ms | ß- + n to 44Cl; ß- to 45Cl | 3/2-# | N/A | 349.32 | - |
| 46S | 46.00075(75)# | 50(8) ms | ß- to 46Cl | 0+ | N/A | 353.67 | - |
| 47S | 47.00859(86)# | 20# ms [>200 ns] | ß- to 47Cl | 3/2-# | N/A | 354.29 | - |
| 48S | 48.01417(97)# | 10# ms [>200 ns] | ß- to 48Cl | 0+ | N/A | 356.78 | - |
| 49S | 49.02362(102)# | <200 ns | n to 48S | 3/2-# | N/A | 356.48 | - |