About Zirconium
Zirconium minerals are very common and have been known to human civilizations for centuries. The first chemist to recognize that the mineral zircon contained a new “earth”--a term early chemists applied to metal oxides--distinct from the already well characterized alumina was Martin Heinrich Klaproth, and he named the new metallic element for its source mineral in 1789. The famous chemist Jons Jacob Berzelius was the first to actually isolate the metal in pure form, a feat accomplished in 1824.
The vast majority of zirconium used commercially is found as some form of zirconium dioxide, also known as zirconia. Zirconia can exhibit three different crystal structures depending on temperature. Unstabilized zirconia may crack when heated or cooled due to transitions between these phases, but stabilized cubic zirconia, produced via addition of other metal oxides, is generally extremely stable across wide temperature ranges. Common forms of stabilized zirconia include magnesia stabilized zirconia, yttria stabilized zirconia, calcia stabilized zirconia, and cerium stabilized zirconia. Stabilized zirconia is frequently used as a refractory ceramic material, in the form of lab crucibles, metal furnaces, thermal barrier coatings, and as a surface coating for foundry molds. It is useful for joining ceramic and metal surfaces, as it has similar thermal expansion properties to steel. Zirconia is also biocompatible, and is frequently used as material for medical implants, either alone, as a coating for metal implants, or in composite metal-ceramic devices. Yttria stabilized zirconia is useful as an electroceramic, found in sensors for detecting conditions such as pH and oxygen levels, and when doped with rare earths can be used for phosphor thermometry. Zirconia is also notable for its ability to conduct ions, which lends it to use as a solid electrolyte in fuel cells.
Zirconium is also a component several other important ceramic materials. Zirconium carbide and zirconium nitride are extremely hard ceramics generally used as refractory materials or cutting tools. Additionally, zirconium is a component of some electroceramics, the most well-known example being lead zirconate titanate, a material used frequently in ceramic capacitors, sensors, and actuators. This compound is essential for the production of ferroelectric RAM, a form of non-volatile random access memory being actively researched by several electronics manufacturers.
A few other zirconium compounds have niche applications as chemical agents. Ammonium zirconium carbonate and potassium zirconium carbonate are used in paper coatings used for the production of high-quality prints. Other zirconium compounds find use as crosslinkers in polymers or in inks to promote adhesion to metals and plastics. Additionally, zirconium hydrides are used as hydrogenation catalysts, reducing agents, foaming agents, and to help produce seals between metals and ceramics.
In metallic form, zirconium is used as an alloying agent. Its primary advantage is high resistance to corrosion, which lends it to use in specialty alloys designed for use in highly corrosive environments. Additionally, zirconium is biocompatible, lending it to use in alloys for biomedical implants, and has a low absorption cross section for thermal neutrons, which dictates its use in nuclear fuel cladding.
Zircon, the source of all zirconium used commercially, is a silicate mineral found as a minor component of heavy mineral sands, which also contain the source minerals for titanium. The two elements are therefore co-products of the same mining operations. Most zircon is never converted to the pure element, and is instead converted to zirconium dioxide, which is the starting material for most other zirconium products. Zirconium metal is produced via the Kroll process, which requires converting zircon to zirconium tetrachloride, which is then reduced to the metal using magnesium. Zircon is also used directly as an opacifier in decorative ceramics, and large crystals of sufficient quality are cut for use as gemstones.
Products
Zirconium is mainly used as a refractory and opacifier. Heating zirconia initiates a phase transformation process resulting in a solid solution. This solid solution is termed as stabilized zirconia, a valuable refractory.  Zirconium is used to a lesser extent as an alloying agent due to its strong resistance to corrosion.
Zirconium is used to a lesser extent as an alloying agent due to its strong resistance to corrosion.  Zirconium is available as metal and compounds with purities from 99% to 99.999% (ACS grade to ultra-high purity). Metallic forms include pellets, rod, wire and granules for evaporation source material purposes. Zirconium nanoparticles and nanopowders provide ultra-high surface area. Zirconium oxides are available in numerous forms such as powder and dense pellets for such uses as optical coating and thin film applications. Oxides tend to be insoluble. Fluorides are another insoluble form for uses in which oxygen is undesirable such as metallurgy, chemical and physical vapor deposition and in some optical coatings. Zirconium is also available in soluble forms including zirconium chloride, nitrate and acetate. These compounds can be manufactured as solutions at specified stoichiometries.
Zirconium is available as metal and compounds with purities from 99% to 99.999% (ACS grade to ultra-high purity). Metallic forms include pellets, rod, wire and granules for evaporation source material purposes. Zirconium nanoparticles and nanopowders provide ultra-high surface area. Zirconium oxides are available in numerous forms such as powder and dense pellets for such uses as optical coating and thin film applications. Oxides tend to be insoluble. Fluorides are another insoluble form for uses in which oxygen is undesirable such as metallurgy, chemical and physical vapor deposition and in some optical coatings. Zirconium is also available in soluble forms including zirconium chloride, nitrate and acetate. These compounds can be manufactured as solutions at specified stoichiometries.
Zirconium Properties
 Zirconium is a Block D, Group 4, Period 5 element.
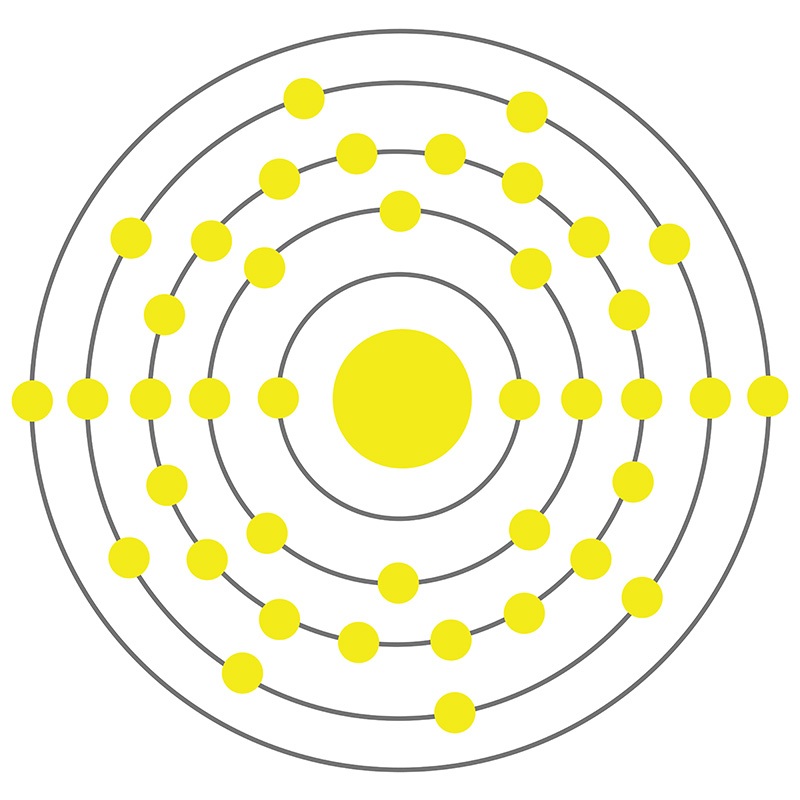
Zirconium is a Block D, Group 4, Period 5 element.  The number of electrons in each of Zirconium's shells is 2, 8, 18, 10, 2 and its electron configuration is [Kr] 4d2 5s2.
The number of electrons in each of Zirconium's shells is 2, 8, 18, 10, 2 and its electron configuration is [Kr] 4d2 5s2.  The zirconium atom has a radius of 159.pm and its Van der Waals radius is 200.pm. In its elemental form, CAS 7440-67-7, zirconium has a silvery white appearance. Zirconium’s principal mineral is zircon (zirconium silicate). Zirconium is produced as a byproduct of titanium and tin mining. It is not found in nature as a native metal. Zirconium was first isolated by Jons Jacob Berzelius in 1824. The name of zirconium comes from the mineral zircon, the most important source of zirconium, and from the Persian word 'zargun' meaning gold color or gold-like.
The zirconium atom has a radius of 159.pm and its Van der Waals radius is 200.pm. In its elemental form, CAS 7440-67-7, zirconium has a silvery white appearance. Zirconium’s principal mineral is zircon (zirconium silicate). Zirconium is produced as a byproduct of titanium and tin mining. It is not found in nature as a native metal. Zirconium was first isolated by Jons Jacob Berzelius in 1824. The name of zirconium comes from the mineral zircon, the most important source of zirconium, and from the Persian word 'zargun' meaning gold color or gold-like.
Health, Safety & Transportation Information for Zirconium
Zirconium is non-toxic, however, safety data for Zirconium and its compounds can vary widely depending on the form. For potential hazard information, toxicity, and road, sea and air transportation limitations, such as DOT Hazard Class, DOT Number, EU Number, NFPA Health rating and RTECS Class, please see the specific material or compound referenced in the Products tab. The below information applies to elemental (metallic) Zirconium.
| Safety Data | |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H250-H251-H260 |
| Hazard Codes | F |
| Risk Codes | 15-17 |
| Safety Precautions | 43-7/8 |
| RTECS Number | ZH7070000 |
| Transport Information | UN 1358 4.1/PG 2 |
| WGK Germany | nwg |
| Globally Harmonized System of Classification and Labelling (GHS) |
|
Zirconium Isotopes
Zirconium has four stable isotopes: 90Zr, 91Zr, 92Zr and 94Zr.
| Nuclide | Isotopic Mass | Half-Life | Mode of Decay | Nuclear Spin | Magnetic Moment | Binding Energy (MeV) | Natural Abundance (% by atom) |
|---|---|---|---|---|---|---|---|
| 78Zr | 77.95523(54)# | 50# ms [>170 ns] | Unknown | 0+ | N/A | 627.18 | - |
| 79Zr | 78.94916(43)# | 56(30) ms | ß+ + p to 78Sr; ß+ to 79Y | 5/2+# | N/A | 640.85 | - |
| 80Zr | 79.9404(16) | 4.6(6) s | ß+ to 80Y | 0+ | N/A | 657.31 | - |
| 81Zr | 80.93721(18) | 5.5(4) s | ß+ to 81Y; ß+ + p to 80Sr | (3/2-)# | N/A | 668.19 | - |
| 82Zr | 81.93109(24)# | 32(5) s | ß+ to 82Y | 0+ | N/A | 681.85 | - |
| 83Zr | 82.92865(10) | 41.6(24) s | ß+ to 83Y; ß+ + p to 82Sr | (1/2-)# | N/A | 692.73 | - |
| 84Zr | 83.92325(21)# | 25.9(7) min | ß+ to 84Y | 0+ | N/A | 705.46 | - |
| 85Zr | 84.92147(11) | 7.86(4) min | ß+ to 85Y | 7/2+ | N/A | 715.41 | - |
| 86Zr | 85.91647(3) | 16.5(1) h | EC to 86Y | 0+ | N/A | 728.14 | - |
| 87Zr | 86.914816(9) | 1.68(1) h | EC to 87Y | (9/2)+ | N/A | 738.09 | - |
| 88Zr | 87.910227(11) | 83.4(3) d | EC to 88Y | 0+ | N/A | 749.89 | - |
| 89Zr | 88.908890(4) | 78.41(12) h | EC to 89Y | 9/2+ | N/A | 759.83 | - |
| 90Zr | 89.9047044(25) | STABLE | - | 0+ | N/A | 771.64 | 51.45 |
| 91Zr | 90.9056458(25) | STABLE | - | 5/2+ | -1.30362 | 778.78 | 11.22 |
| 92Zr | 91.9050408(25) | STABLE | - | 0+ | N/A | 786.86 | 17.15 |
| 93Zr | 92.9064760(25) | 1.5 x 106 y | ß- to 93Nb | 5/2+ | N/A | 794.01 | - |
| 94Zr | 93.9063152(26) | Observationally Stable | - | 0+ | N/A | 802.09 | 17.38 |
| 95Zr | 94.9080426(26) | 64.032(6) d | ß- to 95Nb | 5/2+ | N/A | 808.3 | - |
| 96Zr | 95.9082734(30) | 3.9 x 1019 y | 2ß- to 96Mo | 0+ | N/A | 816.38 | 2.8 |
| 97Zr | 96.9109531(30) | 16.744(11) h | ß- to 97Nb | 1/2+ | N/A | 822.6 | - |
| 98Zr | 97.912735(21) | 30.7(4) s | ß- to 98Nb | 0+ | N/A | 828.81 | - |
| 99Zr | 98.916512(22) | 2.1(1) s | ß- to 99Nb | 1/2+ | N/A | 833.17 | - |
| 100Zr | 99.91776(4) | 7.1(4) s | ß- to 100Nb | 0+ | N/A | 840.31 | - |
| 101Zr | 100.92114(3) | 2.3(1) s | ß- to 101Nb | 3/2+ | N/A | 845.6 | - |
| 102Zr | 101.92298(5) | 2.9(2) s | ß- to 102Nb | 0+ | N/A | 853.68 | - |
| 103Zr | 102.92660(12) | 1.3(1) s | ß- to 103Nb | (5/2-) | N/A | 861.75 | - |
| 104Zr | 103.92878(43)# | 1.2(3) s | ß- to 104Nb | 0+ | N/A | 869.83 | - |
| 105Zr | 104.93305(43)# | 0.6(1) s | ß- to 105Nb; ß- + n to 104Nb | N/A | N/A | 868.6 | - |
| 106Zr | 105.93591(54)# | 200# ms [>300 ns] | ß- to 106Nb | 0+ | N/A | 876.67 | - |
| 107Zr | 106.94075(32)# | 150# ms [>300 ns] | ß- to 107Nb | N/A | N/A | 875.44 | - |
| 108Zr | 107.94396(64)# | 80# ms [>300 ns] | ß- to 108Nb | 0+ | N/A | 883.52 | - |
| 109Zr | 108.94924(54)# | 60# ms [>300 ns] | Unknown | N/A | N/A | 891.59 | - |
| 110Zr | 109.95287(86)# | 30# ms [>300 ns] | Unknown | 0+ | N/A | 890.36 | - |